- Home
- Molecular Oncology
MOLECULAR ONCOLOGY

Introduction
Dr T Rajkumar is a Clinician-Scientist, being the Best Out-going student during MBBS and MD [Gen Medicine] from the Madras Medical College [winning 17 prizes and gold medals, in all], and currently the Professor and Head, Department of Molecular Oncology at the Cancer Institute (WIA), Adyar, Chennai which is a Voluntary Charitable Institution and a Comprehensive Regional Cancer Centre. He had joined the Cancer Institute (WIA) after his MD (General Medicine) for his training in DM (Medical Oncology) in 1986 which he successfully completed in March 1988. He was in-charge Medical Oncologist for the solid tumours, developing newer chemotherapy regimens for the management of ovarian cancer, soft tissue sarcomas and osteosarcomas. In October 1991, he was awarded the prestigious Commonwealth Scholarship for training in Oncology in ICRF Laboratory in Royal Postgraduate Medical School, Hammersmith Hospital, London, UK. He worked under Prof. William Gullick in the Molecular Oncology Department of the ICRF Laboratory and completed my PhD in November 1994.
On return from Hammersmith Hospital, with the support of Dr.S.Krishnamurthi and Dr.V.Shanta Founders of the Cancer Institute (WIA), he established the Department of Molecular Oncology in 1994 and commenced work on the common cancers in this part of the world – namely cervical, breast, gastric cancers as well as on hereditary cancers. The Department has been recognised as a Centre of Excellence by the Dept. of Science and Technology [DST], Govt of India. and has received grants from governmental agencies (DST, Dept of Biotechnology, Govt of India [DBT], Indian Council of Medical Research [ICMR]), Private Foundations like Chennai Willingdon Corporate Foundation and International funding from WHO.
He has held administrative positions in this Institution since 1997, first as Deputy Director [Research], being promoted as Scientific Director in Dec 2000 and then as Director and Scientific Director in June 2006, which he held till Oct 2008. During his stint in Administration, he was liaising with the Ministry of Health, Govt. of India since the Institute was a recognized Regional Cancer Centre and with all the funding agencies. He stepped down from Directorship in Oct 2008, but continued to work as Prof and Head, in his Department.
This helped in bringing in major funding from DST and DBT which enabled development of p16 ELISA for cervical cancer screening [Technology transfer done to HLL Life Care Ltd, Trivandrum], biomarkers for detection of ovarian cancers, blood-based biomarkers for early diagnosis of breast cancer and therapeutic peptide for Ewing’s sarcoma treatment. We have developed and characterised Dendritic cell vaccine for treatment of cervical cancer and completed the Phase 1 Clinical trial in recurrent/metastatic cervical cancer. Subsequently, with major funding from DST, we have completed patient recruitment for the Phase 2 double-blind Dendritic cell vaccine trial in stage IIIB cervical cancer in Sept 2019; we have also received funding from ICMR for a similar trial in metastatic/recurrent ovarian cancers who have failed 2 systemic therapies and the first 4 patients have been recruited.
He had been successful in obtaining research funds for the Department of Molecular Oncology from DST, DBT, ICMR, WHO and Chennai Willingdon Corporate Foundation. These were based on projects which were evaluated by Expert Committee Members of the funding agencies.
Going from the latest, the approximate amount received by funding (2019 to 1997) are:
ICMR – in 2019-Role as Principal Investigator – Multi-institutional – total Rs. 98,000,000. For our Department, Rs. 36,000,000
DBT – 2012- Co-Investigator – multi-institutional- for our Department – Rs. 10,000,000
DST – 2010 to 2020 – Principal Investigator – Rs. 350,000,000
DST – 2007 – Principal Investigator – Rs. 43,000,000
ICMR – 2005 – Indo-German collaboration- Principal Investigator – Rs. 2,175,000
WHO India – 2004 – Principal Investigator – Rs. 1,000,000
DBT – 2002 – Principal Investigator – Rs 2,000,000
DBT – 2002 – Principal Investigator – Rs. 2,250,000
DST – 1999 – Principal Investigator – Rs. 1,800,000
IARC – 1998 – Principal Investigator – Rs. 1,000,000
IARC – 1997 – Co-Investigator – multi-institutional- for our Department – Rs. 750,000
Chennai Willingdon Corporate Foundation – 2002 – Towards purchase of Automated DNA Sequencing unit – Rs. 2,000,000.
Chennai Willingdon Corporate Foundation – 2005 – Towards Research project – Principal Investigator – Rs. 1,500,000
TOTAL = Rs. 514,000,000 [Approximately]; USD 6,700,000 [Approximately]; British £ 5,500,000 [Approximately]
He has been able to build a Team of 8 young Faculty Members [including one Clinician who will become a Clinician Scientist] and mentored them as well. The Team has been able to establish International Collaborations in UK [for microbiome in colon cancer] and Australia [multi-omics analysis of colon cancer- awaiting decision on funding]. The Team Members have been provided independent responsibility [Proteomics; Genomics; Epigenomics; Bioinformatics; Cancer Immunology; Animal models] with a mandate for intra-departmental and inter-institutional collaborations.
He had been selected by the Government of India and then by the International Agency for Research on Cancer (IARC) (WHO wing for Cancer), Lyon, France to serve as a Member of the Scientific Council of IARC, Lyon [2012-2015]. He had also been a Member of the International Collaboration of Epidemiological Studies of Cervical cancer. He has been in several Government of India Committees including the Program Advisory Committee in Health Sciences, Dept. of Science and Technology 2004 -2012, 2015-2018, 2018-2022; DBT Chronic disease biology, Technical evaluation committee [2019-2021]; DBT Nanobiotechnology Task Force (2010-2018); DBT’s Review Committee for projects on North East Twinning Program [2013-2020]; Member, PAC for the Domain III: Healthcare Technology (HT) of DST-IMPRINT 2 [2019-2022]; Member, Governing Board, DBT-International Cancer Project [Genome 2017-2019]; Member, Inter-ministerial Expert Committee to finalize the Guidelines for evaluation of nano-pharmaceuticals in India [2019]; Member, ICMR Gene therapy research committee; Chairman, DSMB for HPV Trial of Serum Institute of India; SAC Member for SyMec, Kalyani and RGCB, Trivandrum; Member, Board of Research Studies, TN Dr.M.G.R Medical University [2010 – 2021]. [/read]
Our Vision
Today’s Research is Tomorrow’s Treatment
Dr. S. Krishnamurthi
Our Mission
As a Clinician you will help thousands of Patients during your lifetime; but as a Researcher you will help millions of People in your lifetime.
Dr. S. Krishnamurthi
Emergency Cases
Please feel welcome to contact our friendly reception staff with any general or medical enquiry call us.
Opening Hours
Registration Time
Visiting Hours
Molecular Diagnostic Unit
Molecular Diagnostic Unit
S.NO | TEST PARAMETERS | PLATFORM USED |
1 | BCR-ABL Qualitative Analysis | Real-Time PCR |
2 | BCR-ABL Quantitative Analysis | Real-Time PCR |
3 | EWS-FLI Testing | Reverse Transcriptase-PCR |
4 | BRCA1, BRCA2 & TP53 Gene Panel | Next Generation Sequencing |
5 | Breast Cancer Extended Gene Panel | Next Generation Sequencing |
6 | Cancer Hotspot Gene Panel | Next Generation Sequencing |
7 | Colon & Lung Cancer Gene Panel | Next Generation Sequencing |
8 | Lung RNA Fusion Gene Panel | Next Generation Sequencing |
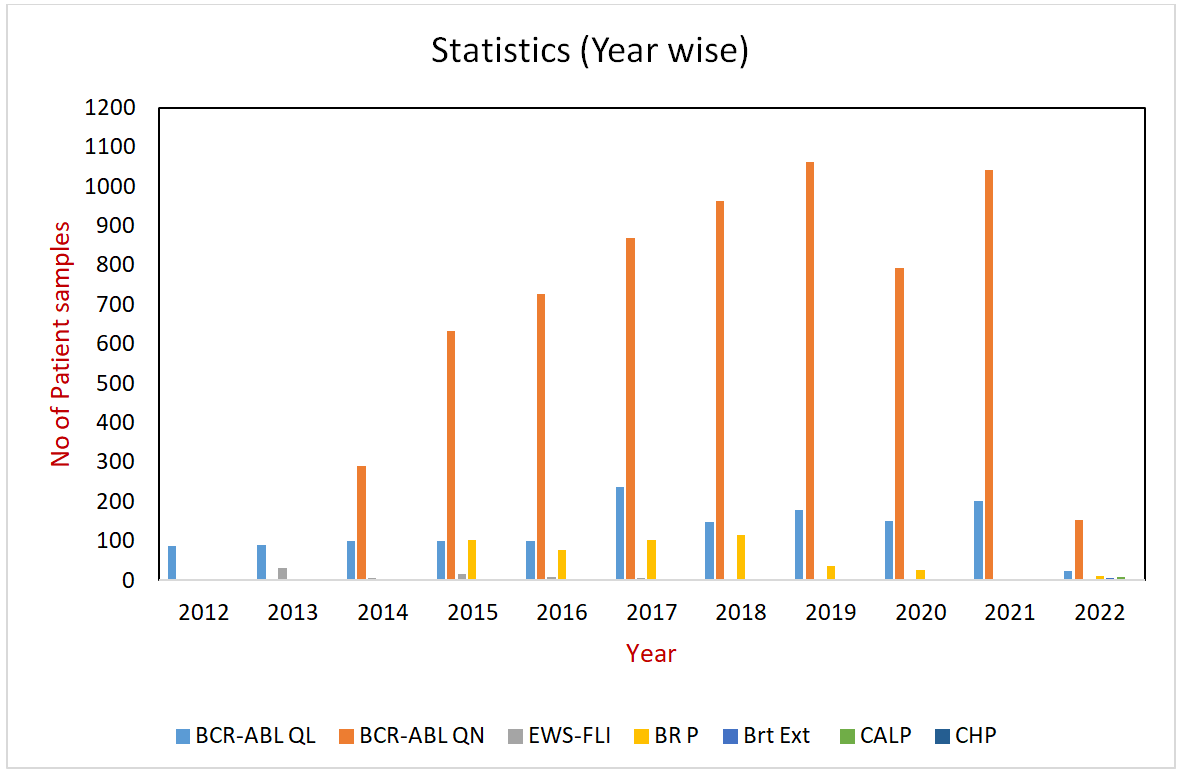
Department Statistics (Year wise)
Research Activities: Ongoing Studies
Ongoing Clinical Trials in the Department
Centre For Advance Research - Development of Dendritic Cell Vaccine for The Treatment of Recurrent/Metastatic Reproductive Cancers (A Multi Institutional CAR) Total Budget: 98,000000 INR (to the Department: 36,000,000 INR)
PI: Dr. T. Rajkumar
Co-Investigators from the Department: Dr. Priya Ramanathan, Dr. Nikita Mehra, Dr. J. Hascitha
Funding agency: Indian Council of Medical Research
Efficacy of Pioglitazone in Patients with Recurrent/ Refractory Inoperable High-Grade Osteosarcoma After Failure of First-Line Chemotherapy: A Pilot Study
PI: Dr. Nikita Mehra
Co-PI from the Department: Dr. T. Rajkumar
Efficacy of Pioglitazone in combination with Neoadjuvant Chemotherapy in Patients with Extremity Skeletal High-Grade Osteosarcoma Treated with Curative Intent: An Open-label, Multi-centre, Randomized Phase II Clinical Trial
PI: Dr. Nikita Mehra
Co-PI from the Department: Dr. T. Rajkumar
Other Projects in the Department
Center For Advance Research - Development of Dendritic Cell Vaccine for The Treatment of Recurrent/Metastatic Reproductive Cancers (A Multi Institutional CAR) Total Budget: 98,000000 INR (to the Department: 36,000,000 INR)
PI: Dr. T. Rajkumar
Co-Investigators from the Department: Dr. Priya Ramanathan, Dr. Nikita Mehra, Dr. J. Hascitha
Funding agency: Indian Council of Medical Research
Phase 2 dendritic cell vaccine clinical trial for stage IIIB cervical cancer
Total Budget: 98,000000 INR (to the Department- 36,000,000 INR)
PI: Dr. T. Rajkumar
Co-Investigators from the Department: Dr. Priya Ramanathan
Funding agency: Department of Science and Technology, Government of India
Identification of tumor suppressor kinases inactivation by pathogenic genetic variants and analysis of consequential dysregulated signalling in gastric cancer.
PI: Dr. G. Gopal
Co-Investigators from the Department: Dr. Samson Mani, Dr. Balaji Ramachandran
Awarding Agency: Core Research Grant Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India
Total Budget: 65,00,000 INR
Understanding the role of 5-hydroxymethylcytosine in breast cancer
PI: Dr. Samson Mani
Co-Investigators from the Department: Dr. T. Rajkumar, Dr. Deva Magendhra Rao. A. K.
Funding Agency: Department of Science and Technology, Government of India
Total Budget: 68,00,000 INR
Genomic and whole transcriptomic characterization of colon cancer
PI: Dr. Samson Mani
Co-Investigators: Dr. T. Rajkumar, Dr. Deva Magendhra Rao. A. K.
Funding Agency: Department of Science and Technology, Government of India
Total Budget: 85,00,000 INR
Project Title: Prognostic implications of circulating non-coding RNAs (lncRNAs/miRNAs) in plasma of breast cancer patients
Principal Investigator: Dr. Samson Mani
Co-Investigators from the Department: Dr. Deva Magendhra Rao. A. K.
OPTIMISTICC (OPportunity To Investigate the Microbiome’s Impact on Science and Treatment In Colorectal Cancer) Large bowel microbiome disease network – Phase 2
Co-Principal Investigator: Dr. Mayilvahanan Bose
Funding Agency: Cancer Research UK
Total Budget: 6,50,000 INR
Development and clinical validation of p16 based ELISA kit for cervical cancer screening.
PI: Dr. Mayilvahanan Bose
Project Co-ordinator: Dr. T. Rajkumar
Funding Agency: DBT-ATGC
Total Budget: 8,91,6634 INR
Modeling of EWS-FLI orthotopic homograft reporter mice
PI: Dr. Balaji Ramachandran
Co-PI: Dr. T. Rajkumar
Co-Investigators from the Department: Dr. G. Gopal
Funding Agency: Core Research Grant Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India
Total Budget: 56,00,000 INR
Studies on Improving the Efficacy of Dendritic Cell Vaccines by targeting Indoleamine 2, 3 Dioxygenases (Ido1 And 2)
PI: Dr. Priya Ramanathan
Co-Investigators from the Department: Dr. Balaji Ramachandran, Dr. Sabitha
Funding Agency: Core Research Grant Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India
Total Budget: 59,60,000 INR
Identification of Aberrant DNA Methylation and Expression of Long non-coding RNAs: Assessing its Functional and Clinical Significance in Monoclonal Gammopathy of Undetermined Significance, Smoldering multiple myeloma and Multiple myeloma Collaborating Institute: Department of Haematology, Christian Medical College, Vellore
PI: Dr. Nikita Mehra
Co-Investigators from the Department: Dr. T. Rajkumar, Dr Samson Mani, Dr Priya Ramanathan
Funding Agency: Core Research Grant Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India
Total Budge: 69,59,000 INR
Detection of M-proteins in Acetonitrile Precipitates of Serum by MALDI-TOF Mass Spectrometry
PI: Dr. Nikita Mehra
Co-Investigators from the Department: Dr. G. Gopal, Mr. S Jayavelu, Dr. T. Rajkumar
Academic/Education activities – Students, courses/training programs conducted routinely
List of Faculty Members
Dr. T. Rajkumar
MD, DM, PhD, DSc, FAMS
Dr. K. Sabitha
MSc, PhD
Dr. G. Gopal
MSc, PhD
Dr. Samson Mani
MSc, PhD
Dr. Balaji Ramachandran
MSc, PhD
Dr. Priya Ramanathan
MSc, PhD
Dr. Mayilvahanan Bose
MSc, M.Phil, PhD
Dr. Deva Magendhra Rao. A. K.
MSc, PhD
Dr. B. Meenakumari
MSc, PhD
Dr. Nikita Mehra
MD, DM
Department Staff Members
Dr. J. Hascitha
MSc, PhD
| Mr. S. Jayavelu | MSc | Scientific Assistant |
| Mr. M. Vijayavel | Diploma, Animal Health Technology | Technologist – Animal House Facility |
| Ms. T. Sangeetha | MSc | Technician (Molecular Diagnostics) |
| Ms. S. Srividya Limkar | MSc | Technician (Molecular Diagnostics) |
| Mr. P. Pitchai Muthu | Diploma, Medical Laboratory | Lab Technician |
| Mr. M. Vijayavel | Diploma, Animal Health Technology | Technologist – Animal House Facility |
Doctoral Students
Ms. S. Amritha | MSc | Senior Research Fellow (Thesis Guide: Dr. T. Rajkumar) |
Ms. S. Abirami | MSc | Senior Research Fellow (Thesis Guide: Dr. T. Rajkumar) |
Ms. R. Deepa | MSc | Senior Research Fellow (Thesis Guide: Dr. Samson Mani) |
Ms. S. Vaishnavi | MTech | Senior Research Fellow (Thesis Guide: Dr. T. Rajkumar) |
Ms. R. Aadhithya | MTech | Junior Research Fellow (Thesis Guide: Dr. G. Gopal) |
Ms. Keerthana Reddy | MSc | Junior Research Fellow (Thesis Guide: Dr. R. Balaji) |
Project Staff
Ms. U. Geethanjali | MSc | Lab Technician (Project PI: Dr. T. Rajkumar) |
Ms. R. Nandhini | MBA | Social Investigator (Project PI: Dr. T. Rajkumar) |
Ms. Sariga | MSc | Technical Assistant (Project PI: Dr. Nikita Mehra) |
Department Publications: January, 2011 to February, 2022
- Mayil Vahanan Bose, Gopisetty Gopal, Ganesharaja Selvaluxmy, Thangarajan Rajkumar* (2012). Dominant negative Ubiquitin conjugating enzyme E2C sensitizes cervical cancer cells to radiation. Int J Rad Biol. 88: 629-634.
2. Priya Ramanathan, Selvaluxmy Ganeshrajah, Rajalekshmi K.R, Shirley Sundar Singh, Thangarajan Rajkumar (2014). Development and clinical evaluation of dendritic cell vaccines for HPV related cervical cancer-a feasibility study. Asia Pacific J Cancer Prev. 15: 5909-5916.
3. Hascitha J, Priya R, Jayavelu S, Dhandapani H, Selvaluxmy G, Sunder Singh S, Rajkumar T. Analysis of Kynurenine/Tryptophan ratio and expression of IDO1 and 2 mRNA in tumour tissue of cervical cancer patients. Clin Biochem. 2016 Aug;49(12):919-24
4. Amutha Periyasamy, Gopal Gopisetty, Velusami Sridevi, Joyimallaya Subramanium Malliga, Thangarajan Rajkumar. Identification of candidate biomarker mass (m/z) ranges in Serous Ovarian Adenocarcinoma using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight mass spectrometry profiling. Biomarkers 2015 20(5):292-8.
5. Amutha Periyasamy and Thangarajan Rajkumar. Role of insulin-like growth factor, insulin-like growth factor receptors and insulin-like growth factor binding proteins in ovarian cancer. IJMPO, 2017; 38(2):198-206
Scientific Meetings
- Workshop on flow cytometry and cell sorting, January 2012
- Advanced flow cytometry and cell sorting workshop, February 2014
- Hands-on training workshop on Liquid Biopsy, October 2016



Innovative Ideas and Practices: List of Patents filed from the Department
- Development of a Double Antibody Sandwich Elisa for detection of p16 in cervical smear lysates. Patent has been filed [Application number 475/CHE/2014, filed on 03-02-2014]. We are in the process of transfer of Technology to a Govt. of India subsidiary company. This kit will help enable population based cervical cancer screening in a cost-effective manner without the need for highly trained Pathologists or Technologists. It can be done at the point off care such as a Primary Health Centre.
- Novel cell penetrating anti-tumorigenic polypeptide for potential treatment of Ewing’s sarcoma. Patent has been filed [Application number 386/CHE/2015].
- Biomarkers for early diagnosis of ovarian cancer Patent filed [Application number 201741011879]
- Blood based biomarkers for early diagnosis and follow up of breast cancer. Patent filed [Ref no: 202041030017; Application number: TEMP/E-1/33382/2020-CHE; PCT Application no: PCT/IN2020/050942].
- A Non - invasive diagnostic and prognostic biomarkers for breast cancer and method of detection. Patent Application Number: 202041055348 dated 19/12/2020
- Rapid and Accurate Detection of M-Protein by MALDI-TOF by Reagent-Based Extraction. Patent has been filed [Application number: 202041009443], March 2020
- A Non-invasive diagnostic and prognostic biomarkers for breast cancer and method of detection.
- Patent Application Number: 202041055348 dated 19/12/2020
- A Non-invasive diagnostic and prognostic biomarkers for breast cancer and method of detection. Patent Application Number: 202041055348 dated 19/12/2020
- Development of SARS-COV2 test kit using RT-LAMP method Patent Application Number : 202041051045 dated 24/11/2020
Quick Contacts
Please feel free to contact our friendly staff with any medical enquiry.
- Emergency Line: (044) 2220 9150
- No:38, Sardar Patel Road, Adyar, Chennai - 20
- Registration Time - 7AM to 11AM

