- Home
- Microbiology
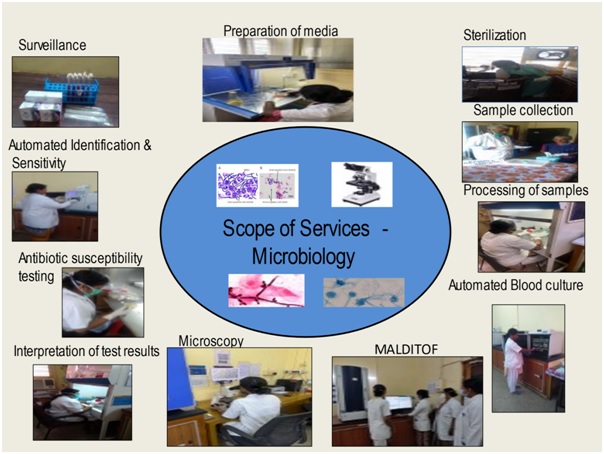
MICROBIOLOGY
The microbiology department provides comprehensive bacteriological, fungal, mycobacterial culture and serological diagnostic service helping in treatment of patients with infection.

Introduction
The microbiology department provides comprehensive bacteriological, fungal, mycobacterial culture and serological diagnostic service helping in treatment of patients with infection. A wide range of samples like blood, body fluids, urine, pus, respiratory secretions, pre op swabs, catheter tips, tissues, stool etc. are received for culture, around 40,000 clinical samples and around 12,000 surveillance samples per year.
Diagnostics
The department has BacT alert automated systems – 120 cells for blood culture specific for adults & paediatric patients thereby helping reduce the turnaround time and results being available after a shortened incubation period of four hours instead of the conventional 24 hours, Mycobacterial culture done using BacT alert automated systems – 60 cells, Automated Vitek 2 Compact system used for identification and susceptibility testing of bacteria and fungus. Anaerobic, Fungal, and Mycobacterial culture of various clinical samples are routinely performed.
MALDI TOF is introduced for early identification of bacteria in blood cultures, urinary tract infections (UTIs), cerebrospinal fluids, respiratory tract infections, stool samples etc. The MALDI Biotyper System identifies microorganisms using MALDI-TOF (Matrix-Assisted Laser Desorption / Ionization Time of Flight) mass spectrometry to determine a unique proteomic Mass fingerprint of an organism.
The department has set its Quality Objective -
- To improve the turn-around time of Gram stain smears from 3-4 hours to 2 hours during Night duty hours
- To reduce the deviations in turn-around time of Blood cultures from ICU / BMT &
- NIL Variance in EQAS report
Surveillance
The department has been helping in monitoring antibiotic resistance, preventing spread of infections, actively involved in surveillance of theatres, Robotic OT, ICU’s, BMT unit, various wards, CSSD, health care personnel and biomedical waste management for better infection control & conducts continued education for nursing and paramedical staff.
Infection Control
It is a major responsibility of the department, regular review of infections and antibiotic policy to limit the spread of infections & resistance is being done. Following the Institute policy of providing a safe hospital environment for our patients, we ensure adherence of strict infection control practice at all levels of the hospital. Relevant cultures are sent at the first suspicion of infection to identify focus and antibiotic initiation is done after sending cultures. Compliance with antibiotic policy is reviewed periodically & all efforts are made to reduce overall antibiotic use. As part of the NABH journey, we have initiated many quality control activities, all the NABH standards pertaining to the laboratory & Infection Control are being adhered at the department. Quality objective set to improve the Hand hygiene compliance from 70% to 80%. WHO – Infection prevention and control assessment tool & Hand hygiene self-assessment framework are being followed. Surveillance of CLABSI, VAP, SSI & CAUTI is done.
Emergency Cases
Please feel welcome to contact our friendly reception staff with any general or medical enquiry call us.
Opening Hours
Registration Time
Visiting Hours
Infrastructure
- Pneumatic chute system in sample receiving area
- 4 Laminar Air Flow Workstations separate for culturing Bacterial, Fungal, Mycobacterial patient samples & for media preparation
- Facility for Fungal Culture with controlled temperature
- Designated area for Hospital Infection Control Activities
- Decontamination area
- Sterilisation area
- Media & Reagent Preparation area
- Serology laboratory
- Hospital Information System for Reporting
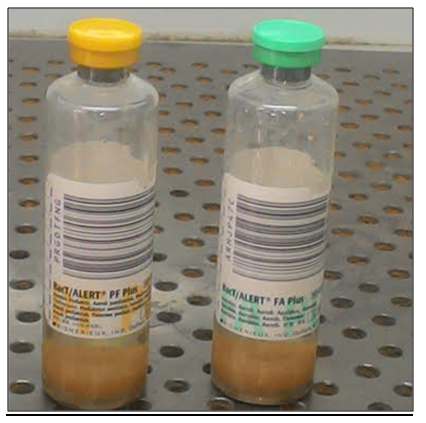
BacT – Alert - Automated blood culture system
a qualitative Microbial detection system used for enhanced recovery and detection of aerobic and facultative microorganisms (Bacteria & fungi) from Blood.
Applications
- The BacT /Alert Microbial detection system is used to determine if microorganisms are present in blood taken from a patient suspected of having Bacteremia / Fungemia
- The BacT /Alert system culture bottles provide both a microbial detection system and a culture medium with suitable nutritional & environmental conditions for organisms commonly encountered in blood infections
Principle
- The BacT / Alert utilize a colorimetric sensor and reflected light to monitor the presence and production of Carbon dioxide (Co2) dissolved in culture medium
- If Microorganisms are present in the test sample, CO2 is produced, as the organisms metabolize the substrates in the culture medium
- When growth of microorganisms produces CO2 the color of the gas-permeable sensor installed in the bottom of each culture bottle changes from blue-green to Yellow
- This lighter color results in an increase of reflectance units monitored by the system
- Bottle reflectance is monitored and recorded by the instrument ever 10 minutes


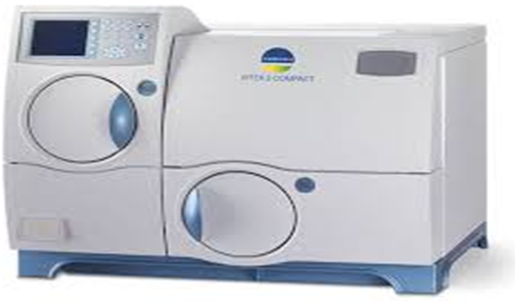

VITEK 2 Automated Identification and Susceptibility testing from Biomerieux, Germany
The VITEK 2 Compact system is dedicated to the identification of bacteria and yeasts and susceptibility testing of clinically significant bacteria.
The system includes the VITEK® 2 Compact instrument, a computer (workstation), and printer.
The software provided with the VITEK® 2 Compact system includes analysis and data-management programs. A bidirectional computer interface transfers results automatically to the user’s laboratory information system (LIS) and to various product and patient reports.
A Quality Control System is available to validate a VITEK® 2 Compact system test kit. An Advanced Expert System™ (Clinical Use) is available to provide online, systematic validation of results and interpretation of resistant phenotypes found during susceptibility testing.
Vitek 2C is automated bacterial and fungal identification and susceptibility system. This is an automated microbiology system utilizing growth-based technology, reducing hands on time for enhanced workflow & rapid reporting. [read more]
The reagent cards have 64 wells that contain an individual test substrate. Substrates measure various metabolic activities such as acidification, alkalization, enzyme hydrolysis, and growth in the presence of inhibitory substances.
An optically clear film present on both sides of the card allows appropriate level of oxygen transmission while maintaining a sealed vessel that prevents contact with the organism-substrate admixtures. Each card has a pre-inserted transfer tube used for inoculation. Cards have bar codes that contain information on product type, lot number, expiration date and a unique identifier that can be linked to the sample either before or after loading the card onto the system.
Inoculated cards are passed by a mechanism, which cuts off the transfer tube and seals the card prior to loading into the carousel incubator. The carousel incubator can accommodate up to 30 cards. All card types are incubated on-line at 35.5 + 1.0ºC. Each card is removed from the carousel incubator once every 15 minutes, transported to the optical system for reaction reading and then returned to the incubator until the next read time. Data are collected at 15-minute intervals during the entire incubation period. [/read]
The MALDI Biotyper System identifies microorganisms using MALDI-TOF (Matrix-Assisted Laser Desorption / Ionization Time of Flight) mass spectrometry to determine a unique proteomic Mass fingerprint of an organism
Principle
• Matrix crystallizes on drying & the sample gets entrapped within the matrix also co-crystallizes.
• The sample within the matrix is ionized in an automated mode with a laser beam generating singly protonated ions from analytes in the sample.
• The protonated ions are then accelerated at a fixed potential, where these separate from each other on the basis of their mass-to charge ratio (m/z).
• The charged analytes are then detected and measured using time of flight (TOF) mass analyzers.
• The m/z ratio of an ion is measured by determining the time required for it to travel the length of the flight tube.
• Based on the TOF information, a characteristic spectrum called peptide mass fingerprint (PMF) is generated for the sample.
• Identification of microbes by MALDI-TOF MS is done by either comparing the PMF of unknown organism with the PMFs contained in the database, or by matching the masses of biomarkers of unknown organism with the proteome database.
Advantages
The pace of diagnostic processes in clinical microbiology laboratories has largely been unchanged for almost 100 years, as availability of diagnostic results essentially depended on the growth of bacteria. Using traditional approaches, it takes at least 24 hours for obtaining growth from clinical specimens and an additional 24 hours for down-stream isolate characterization, as a consequence - therapeutic decisions are generally made empirically until the availability of species identification and resistance patterns.
Techniques providing rapid information on bacterial pathogens and their antimicrobial susceptibility are of key importance for the management of infectious diseases patients. The MALDI Biotyper System identifies microorganisms using MALDI-TOF (Matrix-Assisted Laser Desorption / Ionization Time of Flight) mass spectrometry to deter¬mine a unique proteomic Mass fingerprint of an organism. The introduction of MALDI-TOF into routine diagnostics at Cancer Institute (WIA) led to a significant acceleration of highly specific species identification and we regard this as a major advance in the field of clinical microbiology. MALDI-TOF has considerably reduced the TAT, therefore the slowness of diagnostic procedures prolonging empiric antibiotic therapies are avoided.
Currently, using traditional approaches, it usually takes at least 48 hours for identification and susceptibility testing of bacterial pathogens. Over the years, several new technologies have entered clinical microbiology laboratories, accelerated phenotypic methods, molecular techniques, MALDI -TOF and next generation sequencing etc. MALDI-TOF mass spectrometry fingerprinting has now been widely adopted by clinical microbiology laboratories for rapid identification of cultured microorganisms. Compared to other conventional (e.g. biochemical) identification workflows, turnaround times are typically reduced by at least one working day up to several days for slower growing species or isolates that require complex tests for definite identification. Highest impact on turnaround times and prescription policies is expected for rapid identification from positive blood culture bottles.
Turnaround time - Within half an hour of Growth & Blood culture: Within half an hour of positivity. While MALDI-TOF fingerprinting had originally been introduced and approved for the identification of solid media cultures, it has readily been adopted for liquid enrichment cultures, reduced turnaround times by at least one working day. MALDI -TOF technique is thus suitable to inform clinicians within the critical phase of sepsis, when laboratory reports are known to have highest impact on treatment decisions.
When made available for routine testing, these assays could add to the armamentarium of rapid susceptibility tests needed to reduce time to optimal antimicrobial therapy. With Cancer Institute starting the innovative MALDI TOF Technology for its patients – wee become the 1st Charitable Non-Profit Institution in the State to have this – it makes us proud & we understand that Novel Techniques like this would impact on Antimicrobial Stewardship Program as well.
1. Quality Assurance Manual.
2. Laboratory Safety manual.
3. Standard operating procedures and Policies for each test.
4. Work instructions.
2. External quality assurance
3. Pre – analytic phase
4. Test standardization
5. Post – analytic phase management & organization.
*Corrective & preventive are taken promptly to address any deviations.
hence the following guidelines for prevention & control of COVID-19 were strictly adhered.
Strict implementation of the following are still monitored:
1. Attenders were restricted to 1 per patient by the security staff
2. Wearing of mask, carrying minimal baggage & hand sanitization was made mandatory at the entrance
3. People were scanned through a Thermal scanner & guided to triaging area
4. Social distancing was ensured
5. Biomedical waste disposal and other general infection control measures were monitored
6. Monitoring of OPD, surface cleaning of the chair/ stool/ couches/ tables and other materials after each patient is examined using a checklist was strictly followed
7. Strict monitoring & implementation of the safety measures in the wards was done by Matrons
8. Hand sanitizers were placed in strategic places in both the campuses
9. Bilingual signage in English & Tamil were displayed
2. Applying standard precautions & implementing additional precautions like droplet, contact and airborne precautions.
3. Implementing environmental control, engineering control & administrative controls.
• The support staff engaged in cleaning and disinfection will also wear full complement of PPE.
• Spatial separation of at least 1 meter (3 feet) from one bed to next.
• The access to isolation ward should be through dedicated way.
• Ensure that appropriate hand washing facilities and hand-hygiene supplies are available. Stock the sink area with suitable supplies for hand washing, and with alcohol-based hand rub, near the point of care and the room door.
• Visitors to the isolation ward should be disallowed.


Environmental Cleaning & Disinfection Protocol
Human coronaviruses can remain infectious on surfaces for up to 9 days. COVID-19 virus has been detected after up to 72 hours on plastic and steel, 24 hours on cardboard, 4 hours on copper therefore, cleaning the environment is paramount. Cleaning environmental surfaces with water and detergent and applying commonly used hospital disinfectants (such as sodium hypochlorite) is an effective and sufficient procedure. The following cleaning & disinfection protocol for control & prevention of spread of COVID 19 is strictly being followed in OPD’s, wards, high risk areas – ICU/OT, counter’s, other patient care areas & diagnostic laboratories at both campus.
Many disinfectants are active against enveloped viruses, such as the COVID-19 virus, including commonly used hospital disinfectants.[read more] Sodium hypochlorite & Lysol which is 50% cresol & 50% liquid soap is recommended for spraying. Floor Cleaning with 3 bucket mopping system is followed. High touch surface like doorknobs, door handles, handrails, patient chair rests & arms, lift doors & buttons, sink taps and other fixtures are cleaned by spraying 2.5% Lysol, 70% Isopropyl alcohol to be used to wipe down surfaces where the use of bleach/Lysol is not suitable. Disinfection to be done every 4 hours or more frequently. *Fogging is done using hydrogen peroxide+silver ion MIKROZID HP10 solution after every case & at the end of the list. Cleaning using automated wet mopping machine is done in weekends in above high-risk areas.
Detergent disinfectant B1, B5, B6 products & Harpic are used for toilets, removal of tough stains grease, ramp & corridor washing. Bleaching powder is sprinkled, & 1% hypochlorite is sprayed liberally for disinfection of General waste shed, Bio Medical waste shed & near all manhole, drain holes, drainpipes etc. The commonly used hospital disinfectants Diversy product benzalkonium chloride for floor mopping (Expert/ Swipol) are used first to remove the gross dust & dirt followed by 1% Sodium hypochlorite, 5% Lysol is sprayed in all areas. Pest control is done regularly in external/internal premises of the hospital. Guidelines for handling, treatment, and disposal of COVID-19 Bio Medical Waste are strictly followed.
Collecting & handling of laboratory specimens from patients with suspected COVID-19
All specimens should be regarded as potentially infectious & staff should adhere rigorously to the standard precaution measures and bio safety practices to minimize the possibility of exposure. Soiled linen not for reuse is placed in clearly labelled, leak-proof yellow bags. Decontamination of ambulance, routine cleaning of patient transport bus & staff transport bus regularly done. Disinfection of sump water with chlorination using bleaching powder was also done in regular intervals
Pharmacy: Frequent disinfection of the counter was performed. It was ensured that Attenders / patient wore mask at all times. Training: Training of doctors, nurses, and all Hospital Staff for adapting to current protocols was made mandatory & ongoing.
The department was part of organising Covid Vaccination Camp. [/read]
Services – List, statistics (tables/graphs)
A. Clinical samples
- Microscopy – Gram’s stain, Fungal stain, Ziehl nelson staining etc
- Aerobic culture – Blood, Sputum, Urine, Stool, Pus, Throat swab, Body fluids, CSF etc
- Anaerobic culture – Blood, Body fluids, Pus, Tissue etc
- Fugal culture - Blood, Sputum, Urine, Throat swab, Body fluids, CSF etc
- Mycobacterial culture – Sputum, BAL, Body fluids etc
- Automatic ID/AST using Vitek 2 C
- Automated ID using MALDI TOF
- Serology – ASO, RA, CRP, WIDAL
- Mantoux test
- Clostridium difficle GDH Toxin A & B detection test
B. Passive surveillance of the following
- Operation Theatre
- ICU, BMT, Isolation rooms and wards at both campuses
- Postnasal cultures of OT staff
- Hand cultures of staff in patient care area
- Sterility check of blood bank
- CSSD sterility check of autoclave and ETO
- Water analysis
- Food handlers
| S.NO | SPECIMEN RECEIVED FROM APRIL 2022- MARCH 2023 | No: of specimens |
| 1 | AFB | 155 |
| 2 | AFB Culture | 61 |
| 3 | ASO | 6 |
| 4 | Automated ID/AST and MALDI TOF – all cultures | 2738 |
| 5 | Automated ID/AST (Rapid Culture) | 336 |
| 6 | Ascitic fluid for AFB Culture | 16 |
| 7 | Ascitic fluid for Culture and Sensitivity | 35 |
| 8 | Automated – Central line blood (Blue) for Culture and Sensitivity | 5 |
| 9 | Automated – Central line blood (Green) for Culture and Sensitivity | 3 |
| 10 | Automated – Central line blood (Orange) for Culture and Sensitivity | 1 |
| 11 | Automated – Central line blood (Red) for Culture and Sensitivity | 5 |
| 12 | Automated – Enteric blood for Culture and Sensitivity | 3 |
| 13 | Automated Blood (Central) for Culture and Sensitivity | 683 |
| 14 | Automated Blood (Peripheral) for Culture and Sensitivity | 3207 |
| 15 | Automated Blood for Culture and Sensitivity | 642 |
| 16 | BMA for Culture and Sensitivity | 7 |
| 17 | Bile for Culture and Sensitivity | 33 |
| 18 | Biopsy for Culture and Sensitivity | 7 |
| 19 | Blood Widal test | 29 |
| 20 | Blood for Fungal Culture and Sensitivity | 30 |
| 21 | Bronchial Wash for AFB | 74 |
| 22 | Bronchial Wash for AFB Culture | 77 |
| 23 | Bronchial Wash for Culture and Sensitivity | 148 |
| 24 | CRP | 3643 |
| 25 | CSF for AFB | 43 |
| 26 | CSF for AFB Culture | 29 |
| 27 | CSF for Culture and Sensitivity | 90 |
| 28 | Catheter tip | 13 |
| 29 | Central Line tip | 338 |
| 30 | Clostridium Difficle GDH Toxins A and B | 65 |
| 31 | Culture & Sensitivity | 461 |
| 32 | Culture Of | 3 |
| 33 | ETT Swab for Culture and Sensitivity | 22 |
| 34 | ETT tip | 1 |
| 35 | FNAC for AFB | 7 |
| 36 | Fluid for Culture & Sensitivity | 116 |
| 37 | Fungal Culture | 143 |
| 38 | Fungal stain | 211 |
| 39 | Gram Stain – direct | 17452 |
| 40 | Gram Stain – from samples | 1963 |
| 41 | Mantoux Test | 199 |
| 42 | Other stains | 2 |
| 43 | Pericardial fluid for AFB | 2 |
| 44 | Pericardial fluid for Culture and Sensitivity | 4 |
| 45 | Peritoneal fluid for AFB | 8 |
| 46 | Peritoneal fluid for Culture and Sensitivity | 4 |
| 47 | Pleural fluid for AFB | 73 |
| 48 | Pleural fluid for AFB Culture | 46 |
| 49 | Pleural fluid for Culture and Sensitivity | 146 |
| 50 | Pre OP Swab for Culture | 113 |
| 51 | Pus for Culture and Sensitivity | 117 |
| 52 | Pus swab for Culture and Sensitivity | 144 |
| 53 | RA Factor | 11 |
| 54 | Sputum for AFB X 3 days | 186 |
| 55 | Sputum for AFB Culture | 186 |
| 56 | Sputum for Culture and Sensitivity | 964 |
| 57 | Sputum for Fungal culture | 63 |
| 58 | Sputum in Tracheal Tube | 29 |
| 59 | Stem Cell Culture | 74 |
| 60 | Stool Hanging Drop Method | 6 |
| 61 | Stool for Culture and Sensitivity | 1316 |
| 62 | Throat Swab for Culture and Sensitivity | 19 |
| 63 | Tissue for Culture and Sensitivity | 90 |
| 64 | Urine for Culture and Sensitivity | 3794 |
| 65 | Urine for fungal culture | 6 |
| 66 | Vaginal Swab for Culture and sensitivity | 13 |
| 67 | Wound Swab for Culture and Sensitivity | 396 |
| 68 | Anaerobic cultures | 258 |
| 70 | Operation theater surveillance | 8523 |
| 71 | Ward / personnel | 1900 |
| 72 | CSSD | 918 |
| 73 | ICU/ BMT | 293 |
| 74 | Water analysis | 1624 |
| 75 | Blood bank | 178 |
| Total no of Samples | 54,606 | |
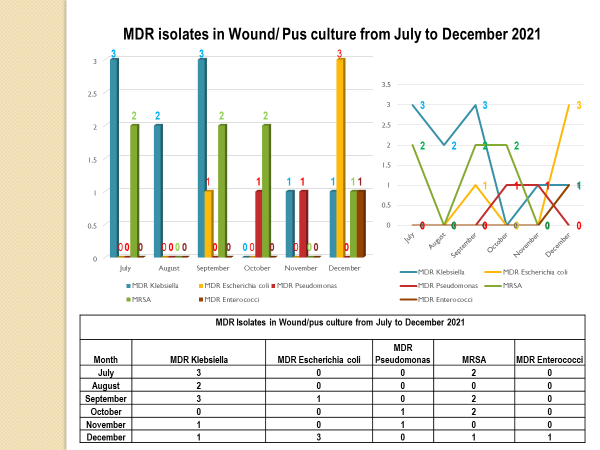
Antibiotic Susceptibility Pattern (SENSITIVITY %) from April 2022 – March 2023
| BLOOD | ||||||||||||
| Organism | I | ME | PT | CS | AK | CFL/ LE | CU/ CI/CE | CL | LZ | VA | TE | TGC |
| Pseudomonas | 96 | 96 | 96 | 96 | 100 | 96 | NA | 100 | NA | NA | NA | NA |
| Staph aureus | 94 | 94 | 94 | 94 | 94 | 94 | – | NA | 100 | 100 | 100 | 100 |
| Klebsiella | 89 | 89 | 89 | 89 | 89 | 45 | 45 | 100 | NA | NA | NA | 100 |
| Escherichia coli | 67 | 67 | 67 | 67 | 75 | 33 | 17 | 100 | NA | NA | NA | 100 |
| WOUND SWAB | ||||||||||||
| Organism | I | ME | PT | CS | AK | CFL/ LE | CU/ CI/CE | CL | LZ | VA | TE | TGC |
| Staph aureus | 92 | 92 | 92 | 92 | 94 | 74 | – | NA | 100 | 100 | 100 | 100 |
| Pseudomonas | 96 | 96 | 96 | 96 | 96 | 94 | NA | 100 | NA | NA | NA | NA |
| Escherichia coli | 98 | 98 | 98 | 98 | 98 | 22 | 13 | 100 | NA | NA | NA | 100 |
| Klebsiella | 85 | 85 | 79 | 79 | 87 | 42 | 27 | 100 | NA | NA | NA | 100 |
| RESPIRATORY SAMPLE | ||||||||||||
| Organism | I | ME | PT | CS | AK | CFL/ LE | CU/ CI/CE | CL | TGC | I | ME | PT |
| Pseudomonas | 97 | 97 | 99 | 97 | 99 | 97 | NA | 100 | NA | 97 | 97 | 99 |
| Klebsiella | 80 | 80 | 79 | 79 | 81 | 52 | 43 | 100 | 100 | 80 | 80 | 79 |
| Escherichia coli | 90 | 90 | 90 | 90 | 90 | 57 | 38 | 100 | 100 | 90 | 90 | 90 |
| URINE | ||||||||||||
| Organism | I | ME | PT | CS | AK | CFL/ LE | CU/ CI/CE | CL | LZ | VA | TE | TGC |
| Escherichia coli | 93 | 93 | 93 | 93 | 95 | 42 | 44 | 100 | NA | NA | NA | 100 |
| Enterococci | 73 | 73 | 70 | 70 | NA | 62 | NA | NA | 100 | 98 | 98 | 100 |
| Pseudomonas | 85 | 85 | 82 | 82 | 85 | 70 | NA | 100 | NA | NA | NA | NA |
| Klebsiella | 84 | 84 | 84 | 84 | 85 | 41 | 43 | 100 | NA | NA | NA | 100 |
Abbreviations used
- I – Imipenem
- ME – Meropenem
- PT – Piperacillin / Tazobactam
- CS –Cefoperazone / Sulbactam
- AK – Amikacin
- CFL/ LE – Ciprofloxacin/ Levofloxacin
- NA – Not applicable
- CU/ CI/ CE – Cefuroxime/ Ceftriaxone/ Cefotaxime
- CL – Colistin
- LZ – Linezolid
- VA – Vancomycin
- TE – Teicoplanin
- TGC- Tigecycline
Standard - HIC 6g - Feedback regarding surveillance data - infection rates / trends in HIS
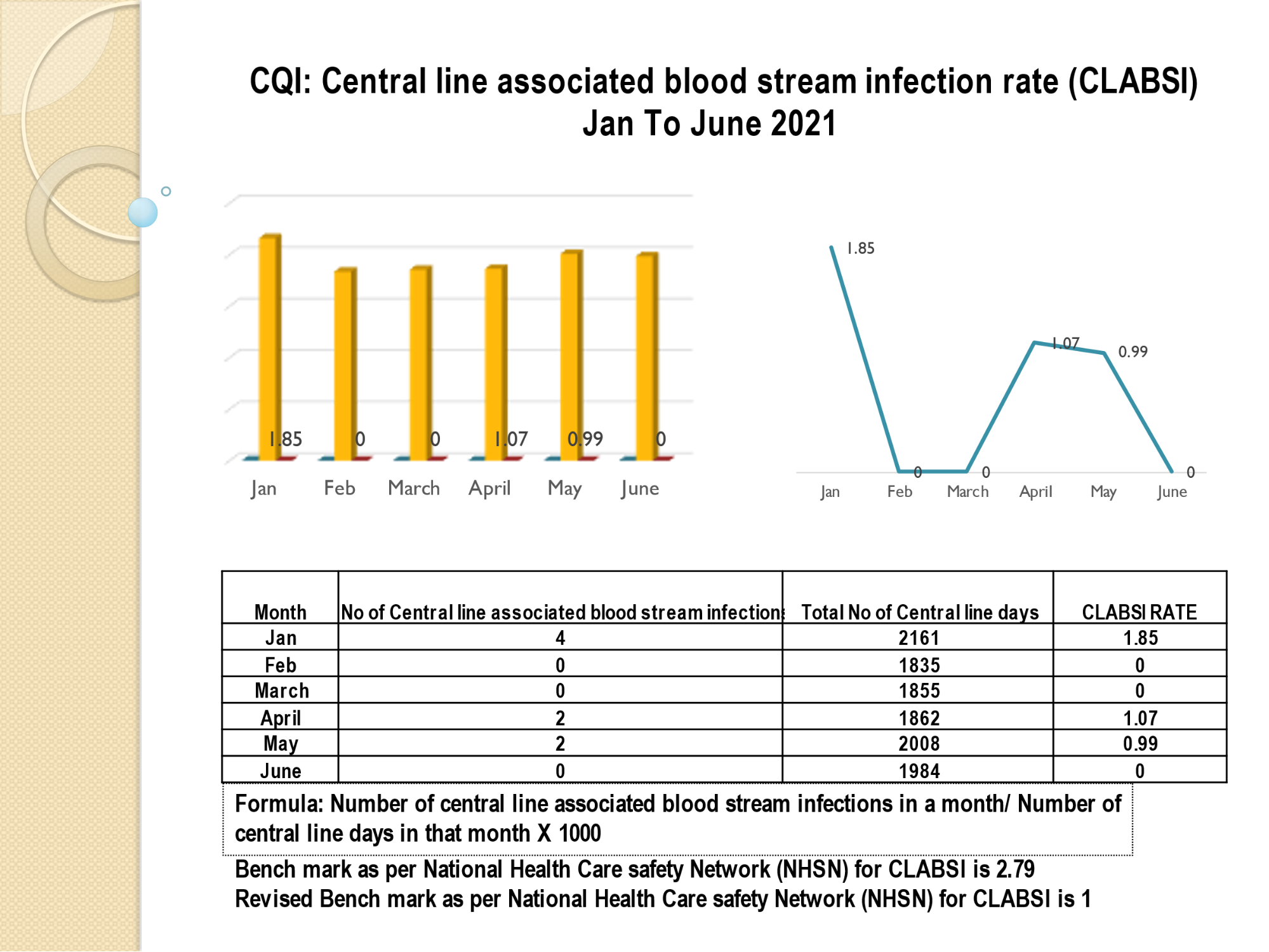
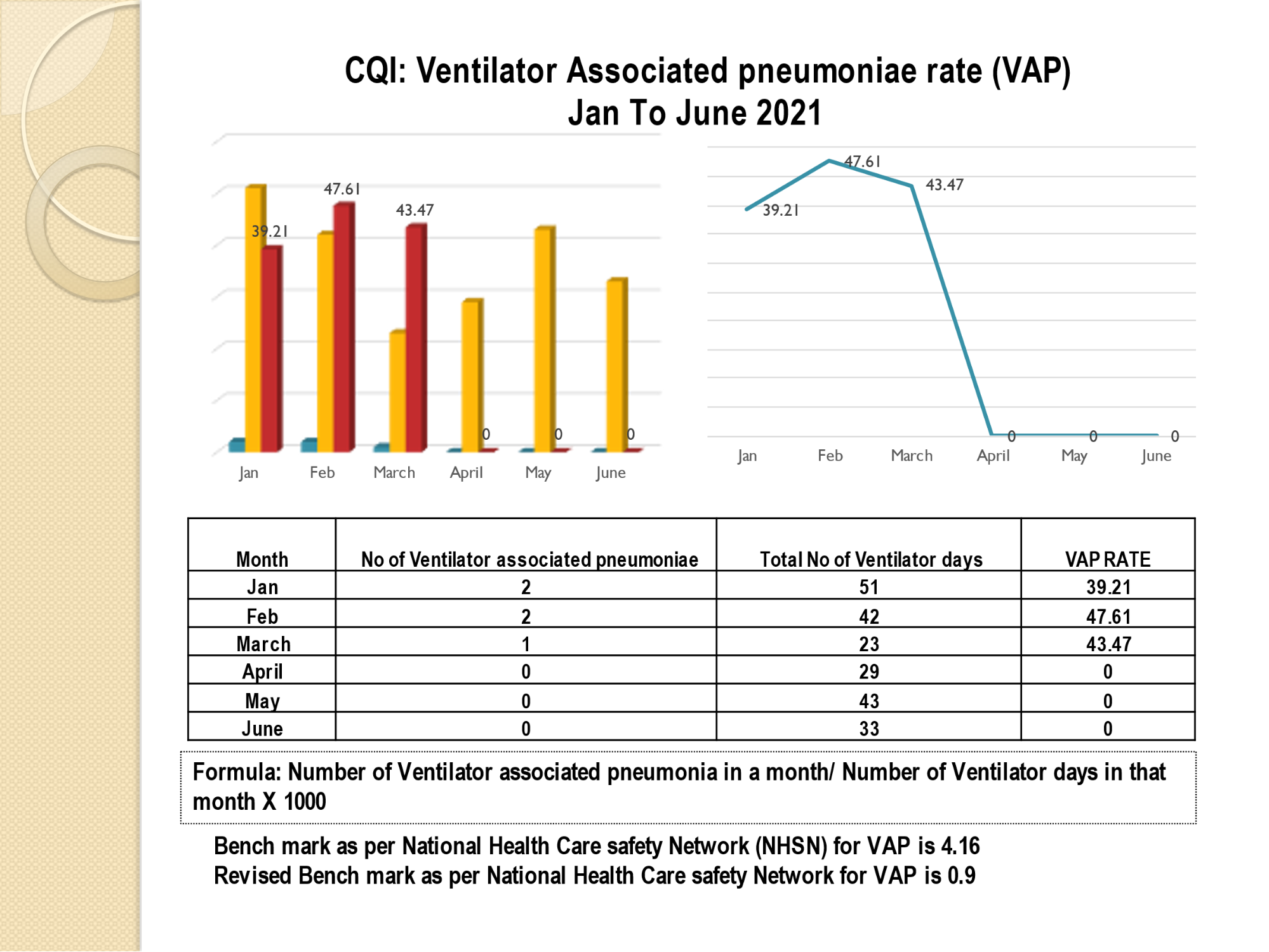
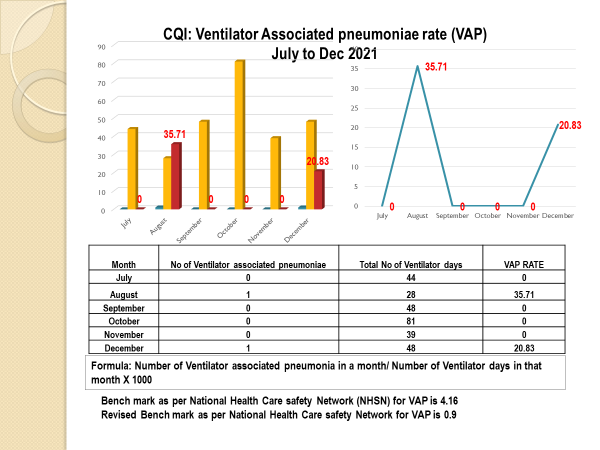
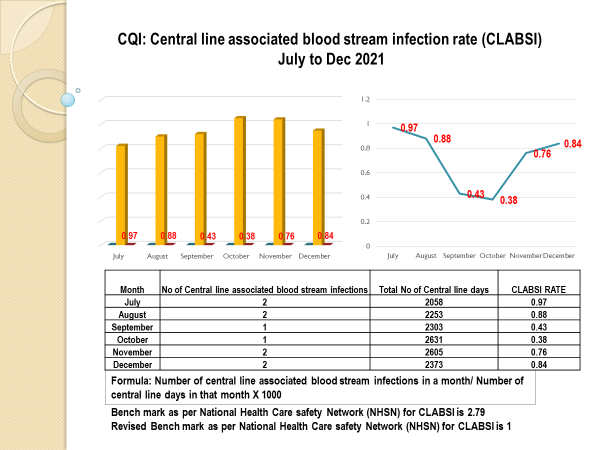
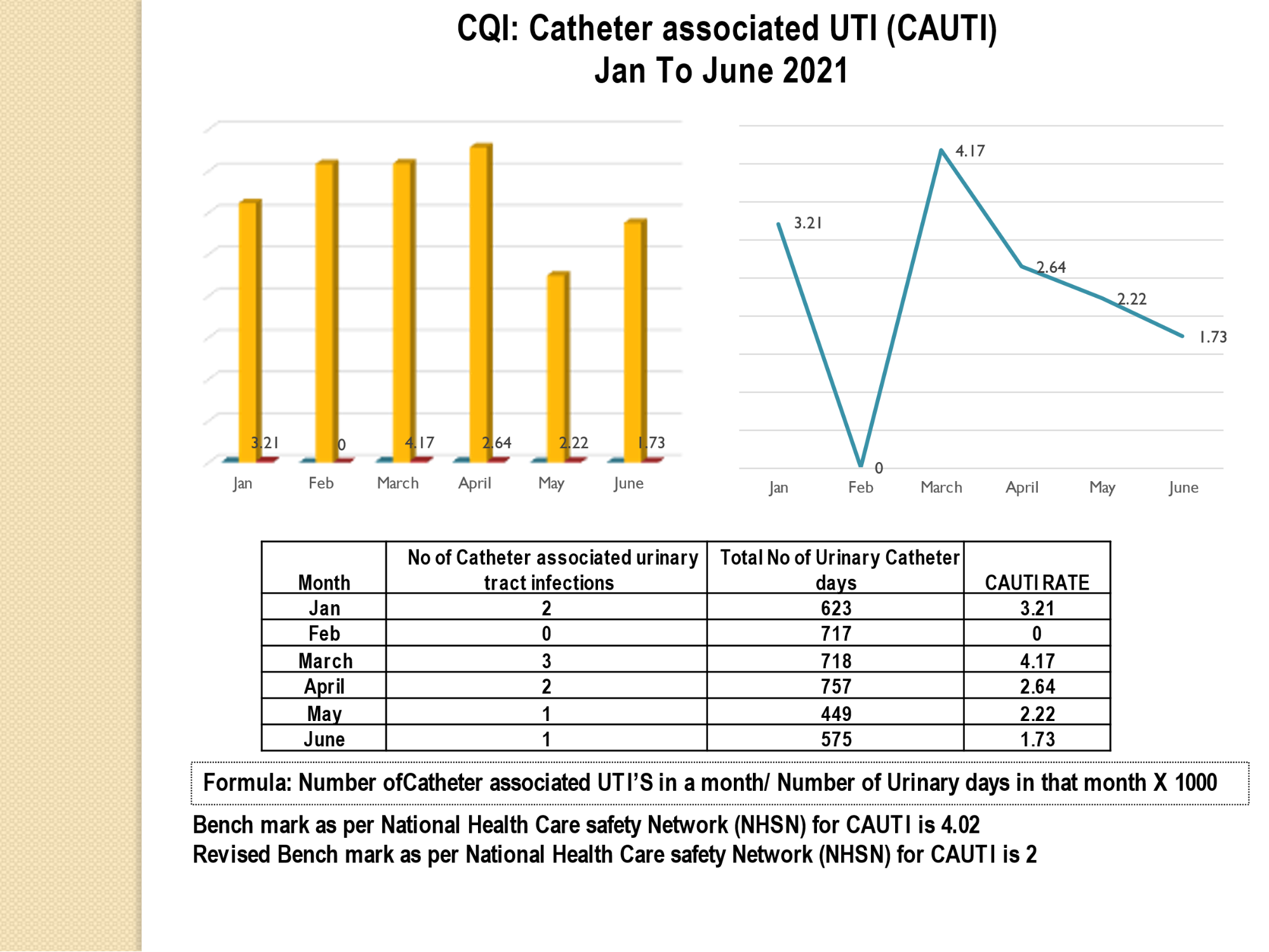
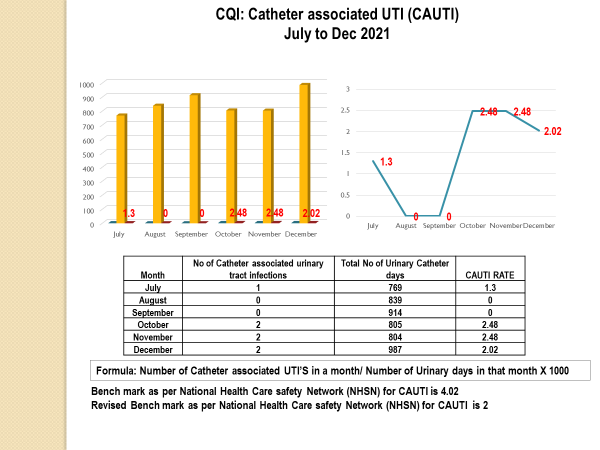
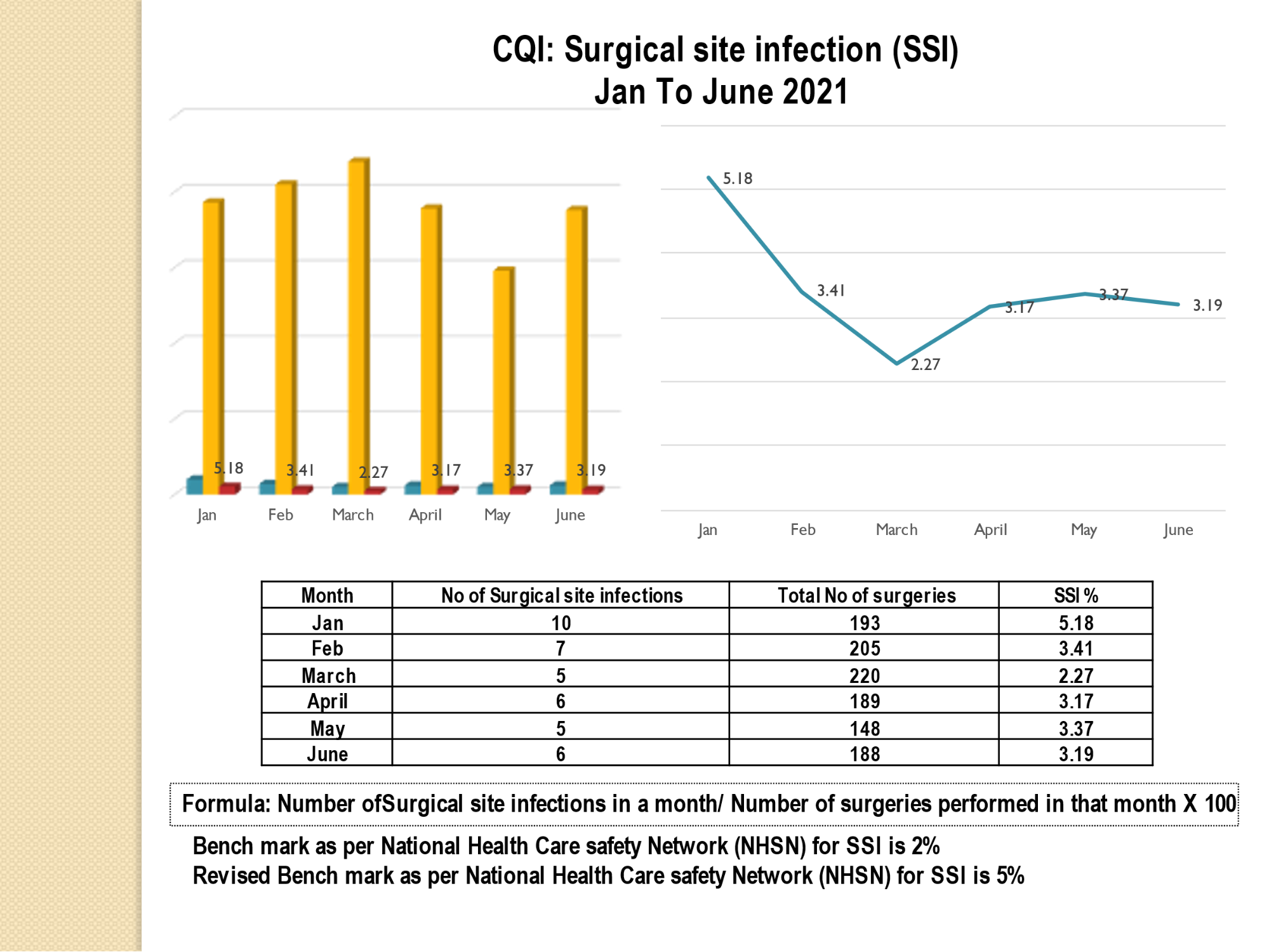
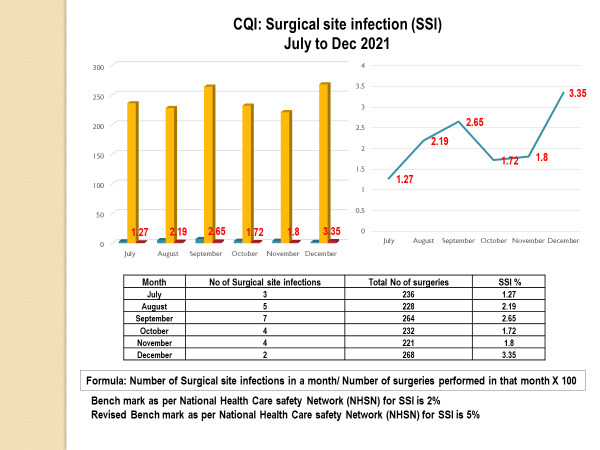
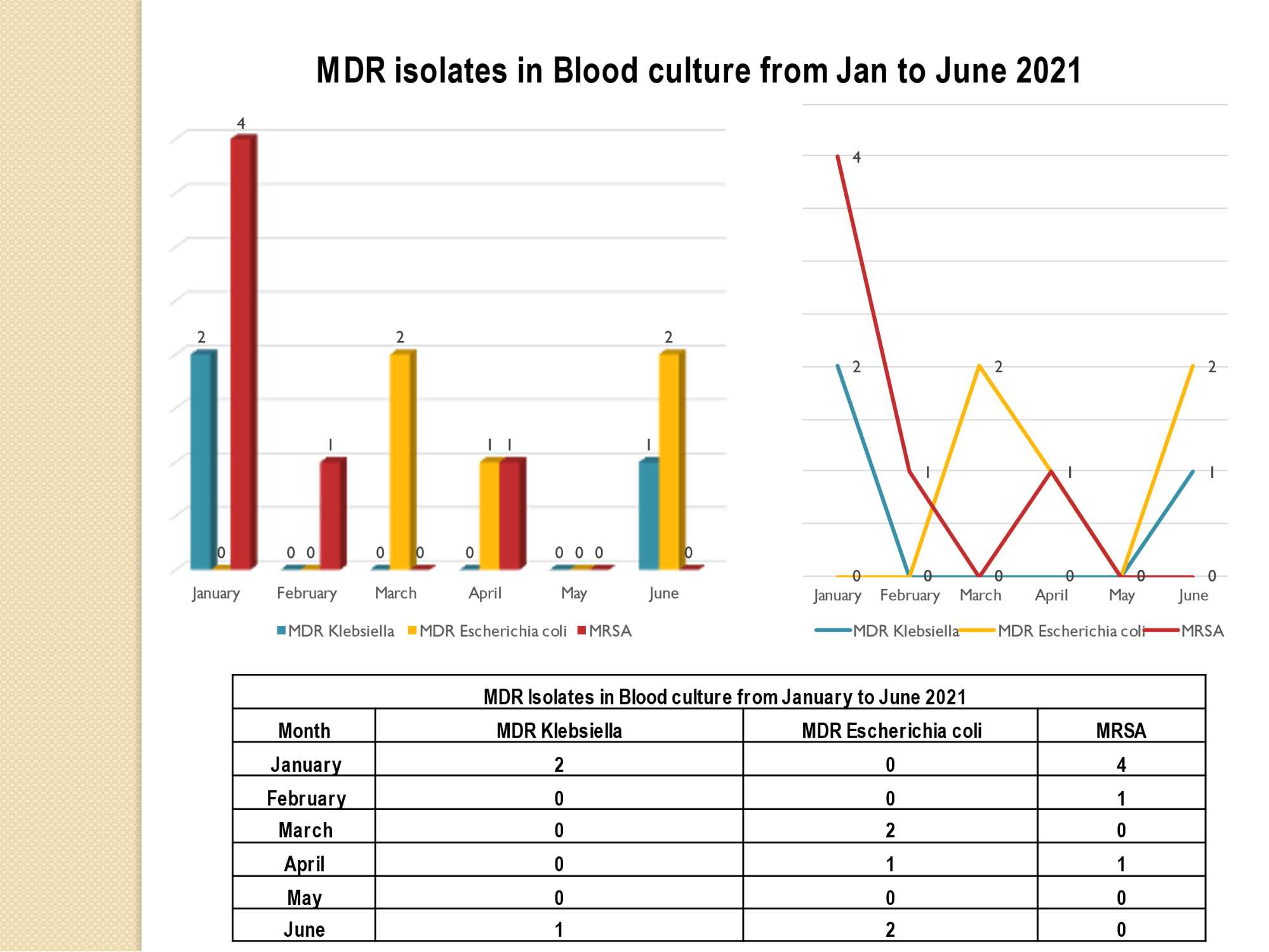
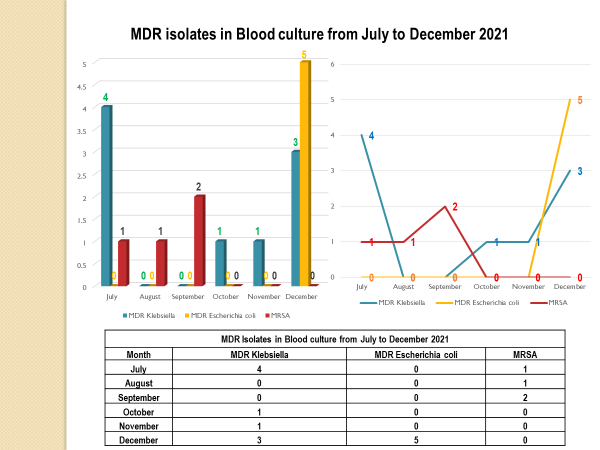
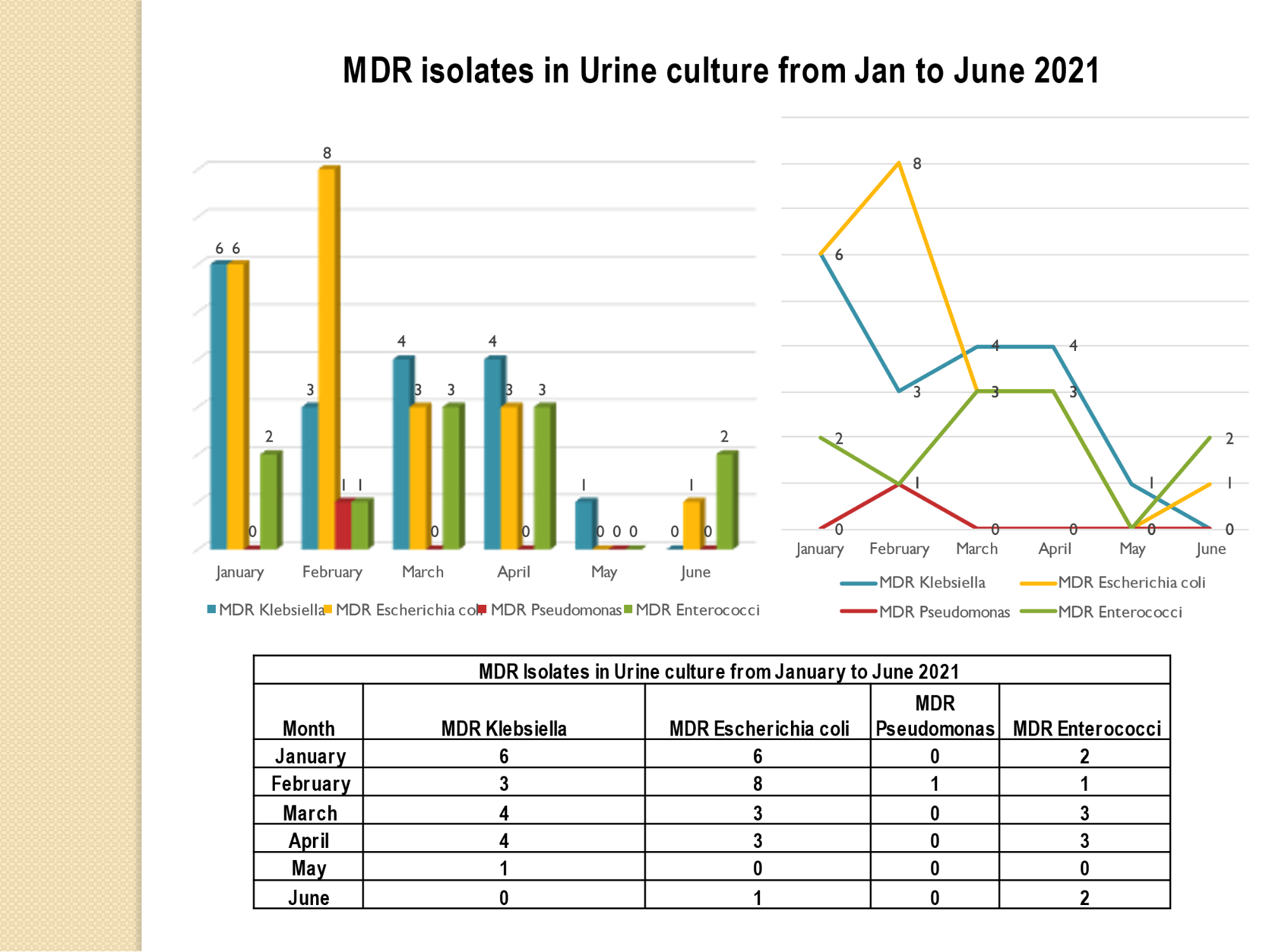
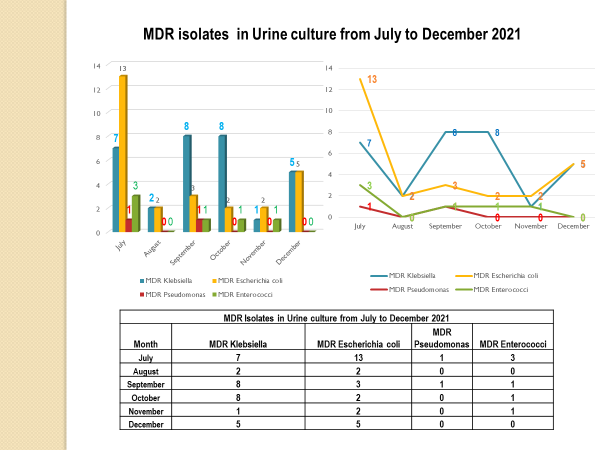
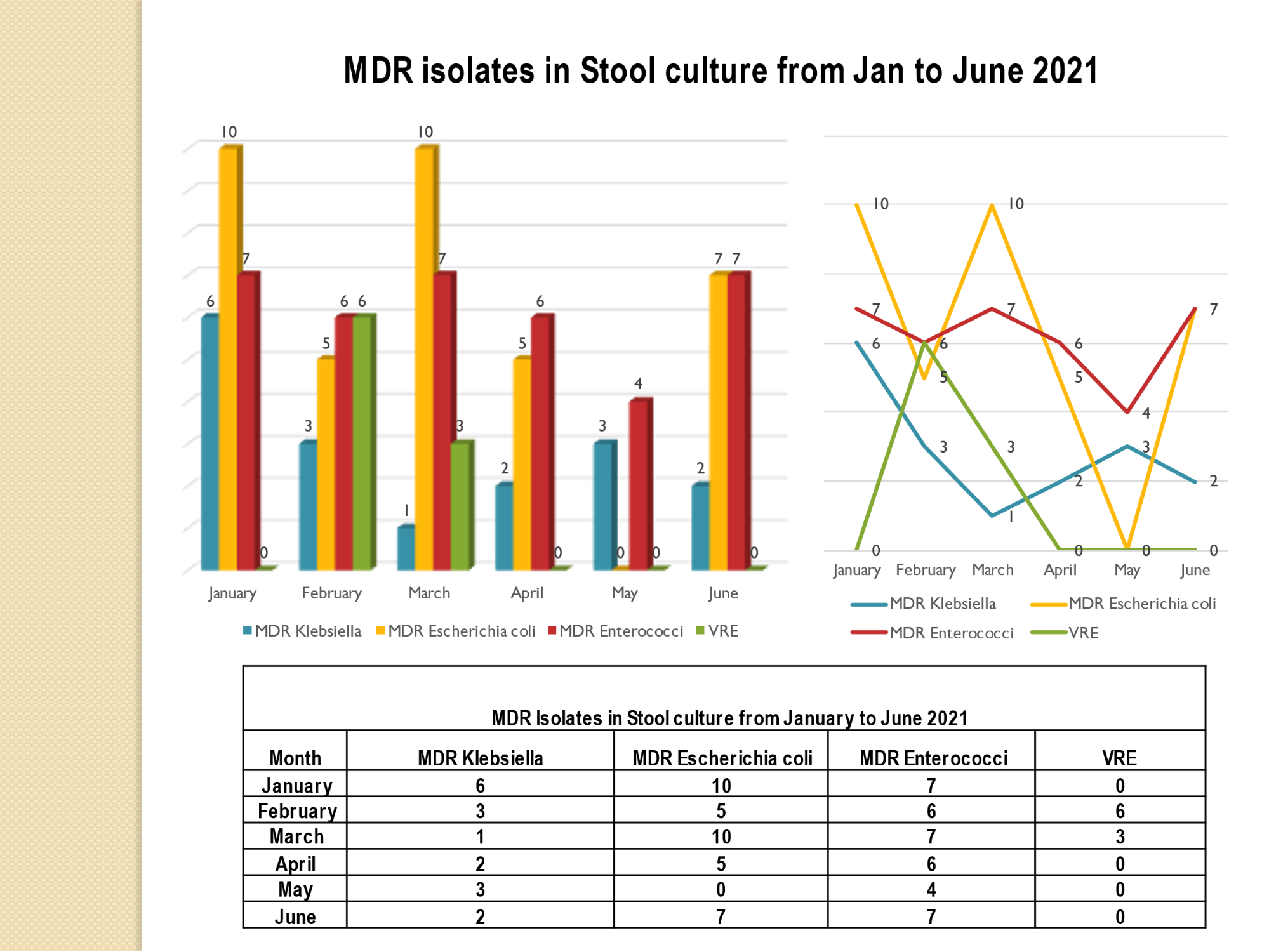
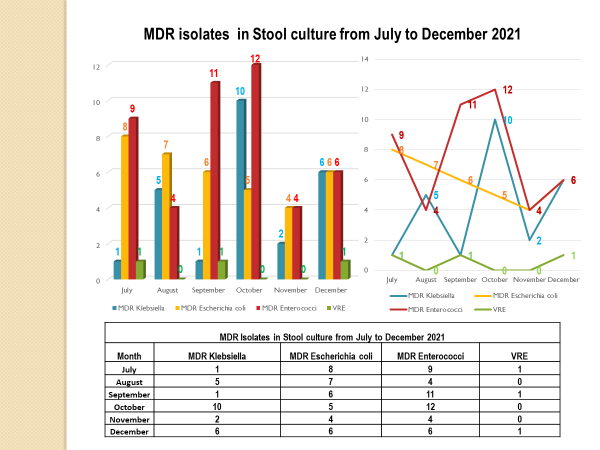
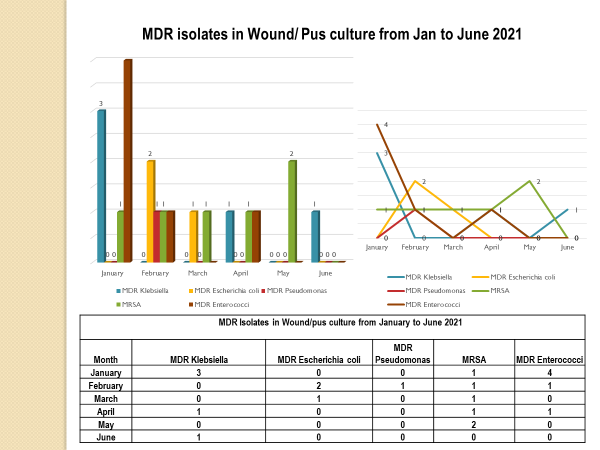
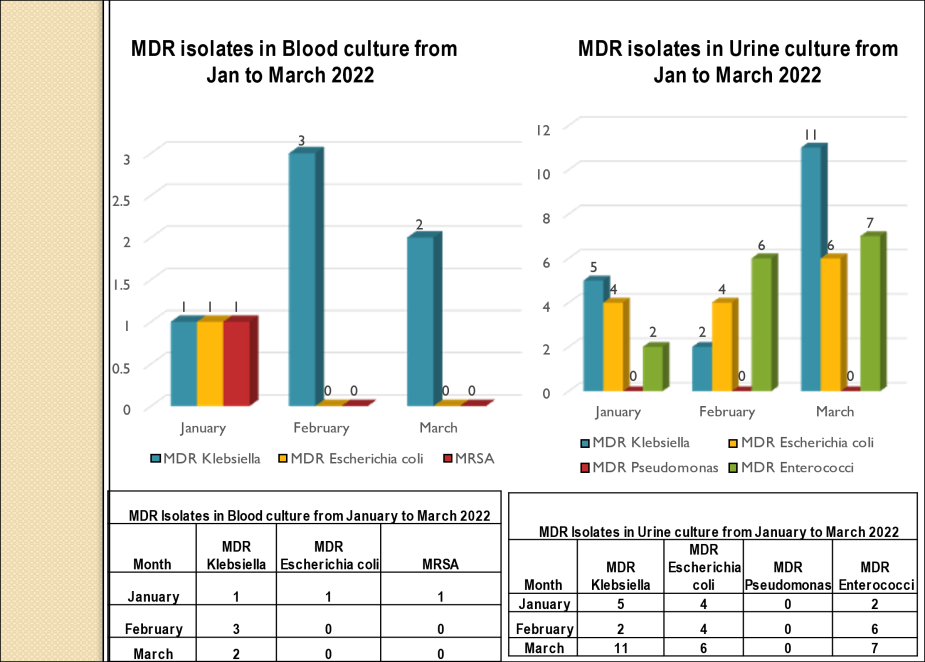
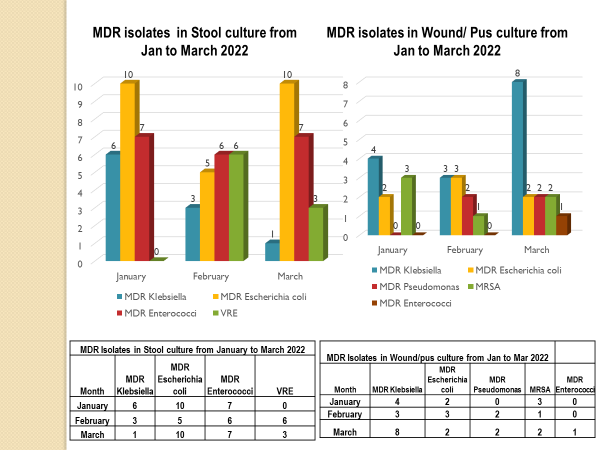
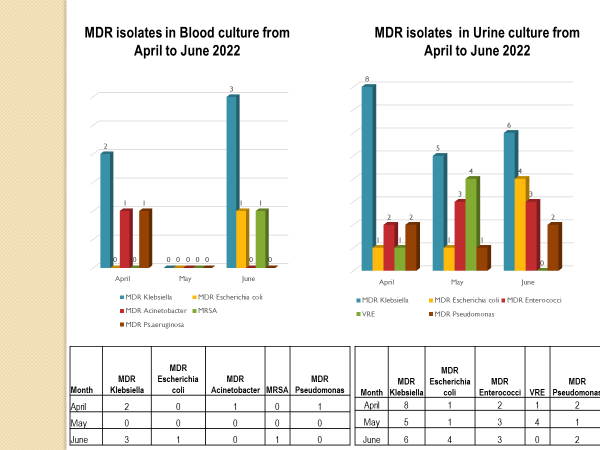
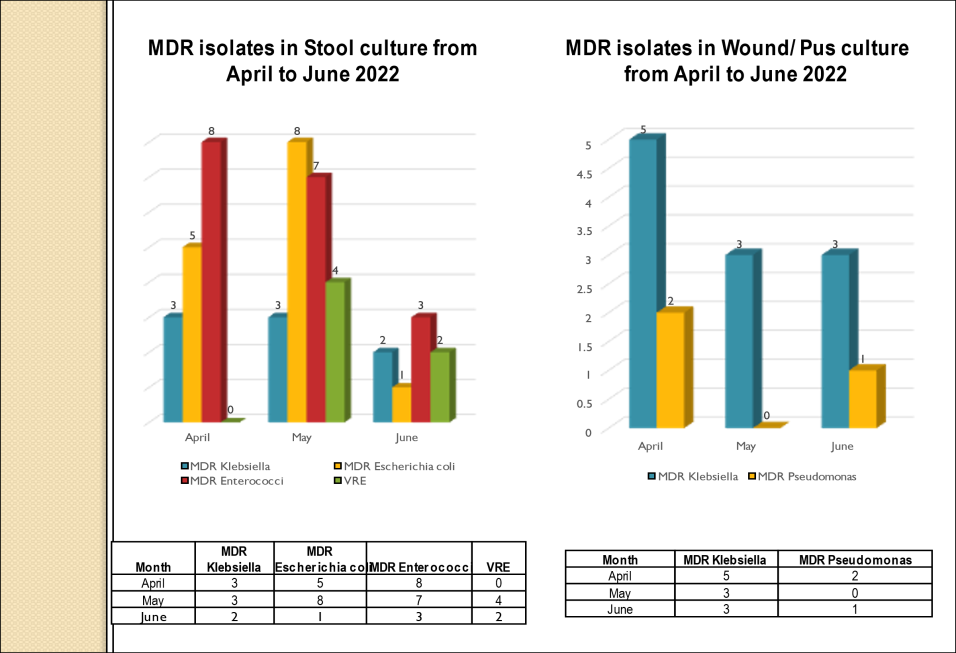
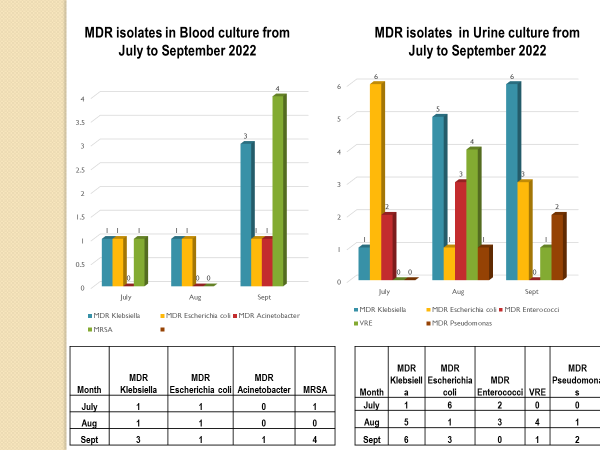
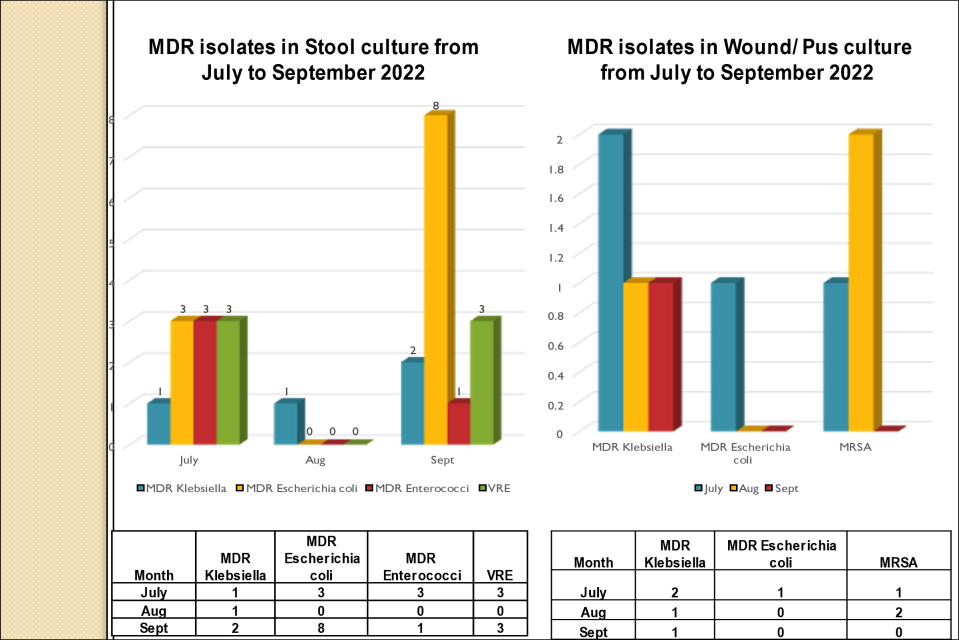
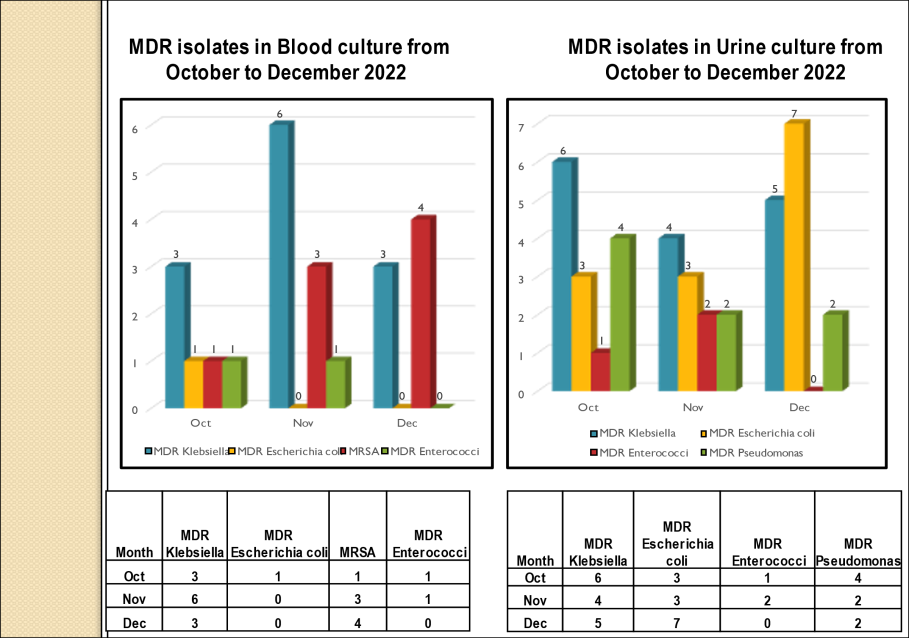
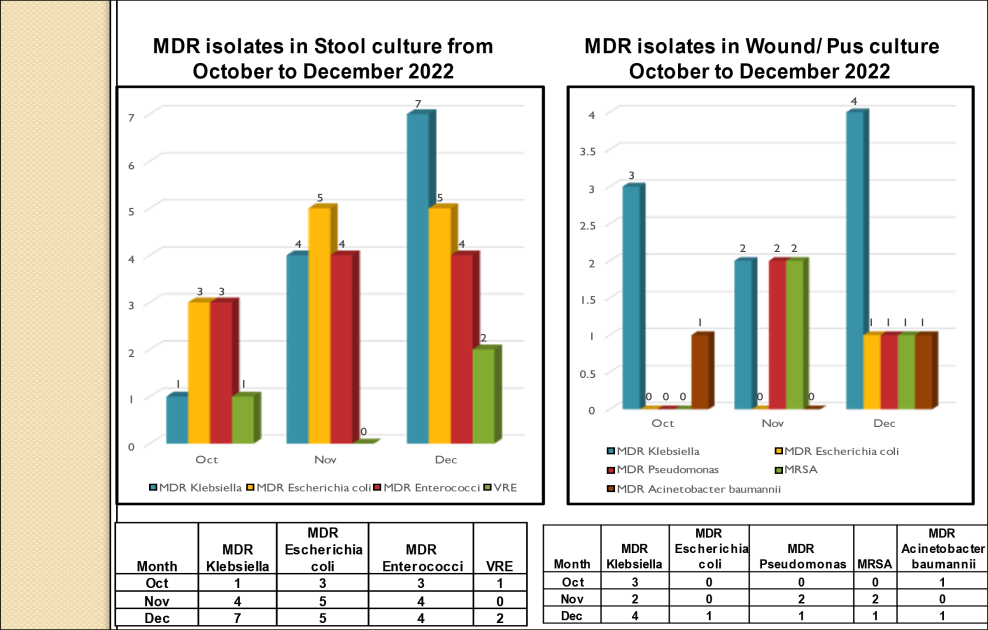
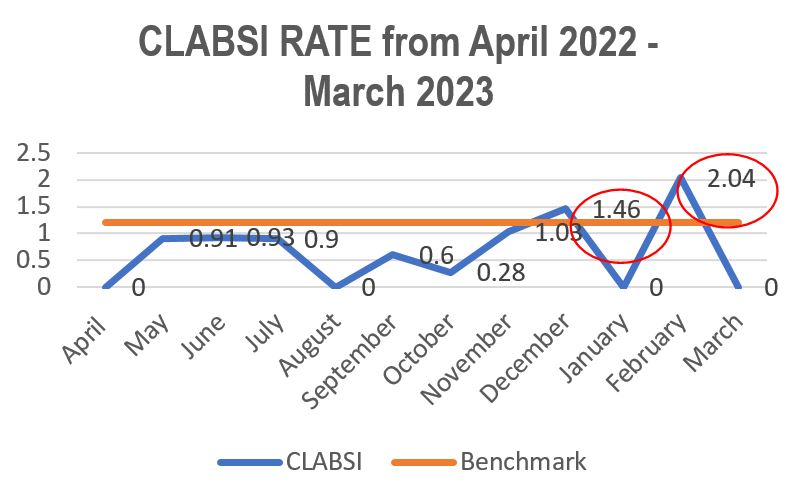
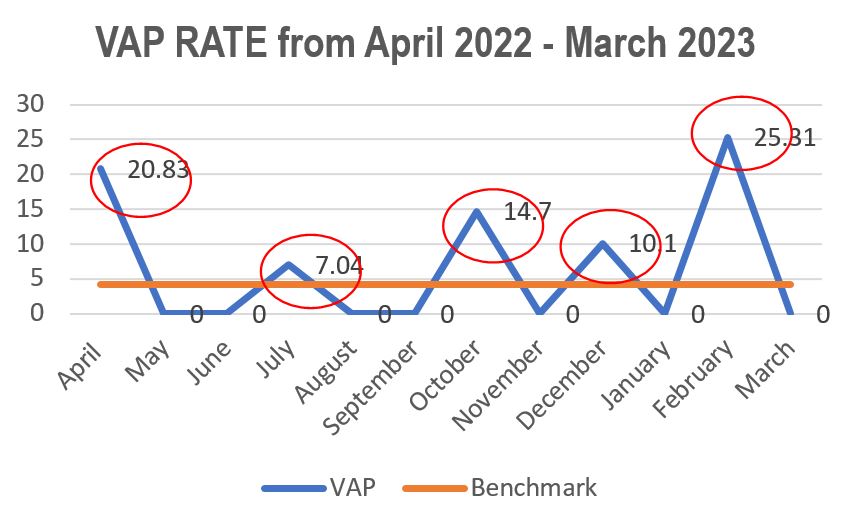
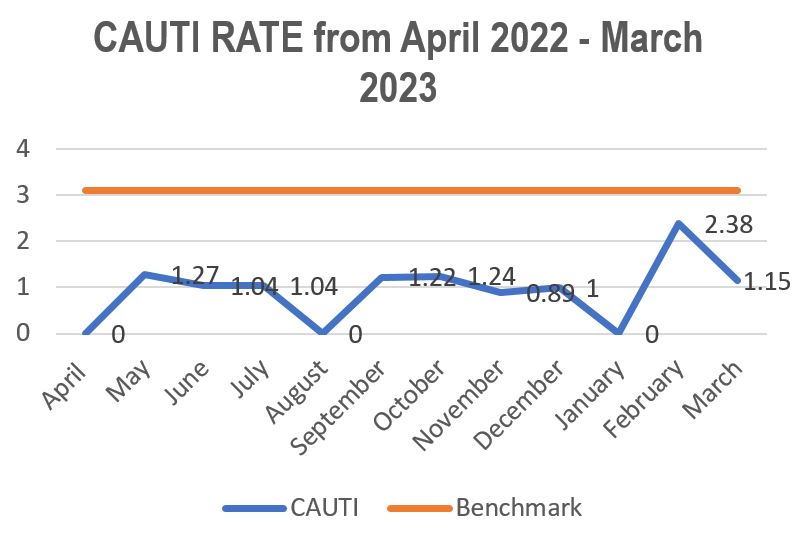
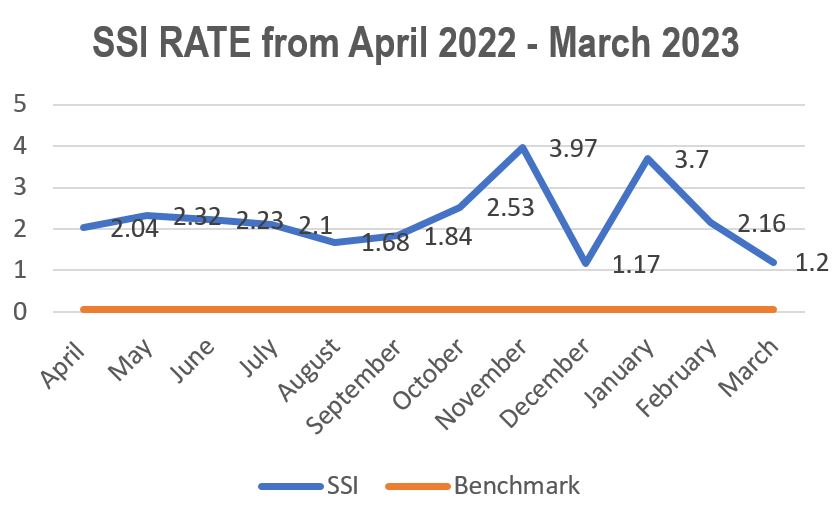

Academic activities
- In service training for all category of staffs.
- Training for students from outside colleges – observership
Staff Members
| 1 | Mrs. Varalakshmi | (M.Sc Medical Micro) | Head, Infection Control Officer, Associate Director(Admin) |
| 2 | Dr. Jennifer Emelda E | (Ph.D., Biomedical science) | Senior Microbiologist |
| 3 | Mrs. Harini | (M.Sc Medical Micro) | Scientific Assistant |
| 4 | Mrs. Saraswathy | (M.Sc Applied Micro) | Scientific Assistant |
| 5 | Ms. Samudhra | (MSc Medical Micro) | Junior Scientific Assistant |
| 6 | Mrs. Valarmathi | (B.Sc, DMLT) | Technician |
| 7 | Mrs. Meena | (B.Sc MLT) | Technician |
| 8 | Mr. Vijayaragavan | (DMLT) | Technician |
| 9 | Ms. Monika | (DMLT) | Technician |
| 10 | Ms. Nandhini | (B.Sc, Applied Micro) | Trainee Technologist |
| 11 | Ms. Aarthishwari | (DMLT) | Trainee Technician |
| 12 | Ms Enisha | (B.Sc, Applied Micro) | Technologist |
Quick Contacts
Please feel free to contact our friendly staff with any medical enquiry.
- Emergency Line: (044) 2220 9150
- No:38, Sardar Patel Road, Adyar, Chennai - 20
- Registration Time - 7AM to 11AM

