Established in 1994, the Department of Molecular Oncology was founded with the guiding mission: “Today’s Research, Tomorrow’s Cure.”

Established in 1994, the Department of Molecular Oncology was founded with the guiding mission: “Today’s Research, Tomorrow’s Cure.” Recognized as a Centre of Excellence by the Department of Science and Technology (DST), Government of India, the department has received significant research funding from prominent national agencies including DST, DBT, ICMR, BRNS, and the DBT-Wellcome Trust.
The department focuses on both fundamental and translational cancer research, with the aim of advancing scientific understanding and improving therapeutic approaches. It is equipped with state-of-the-art instrumentation such as the Berthold in vivo imaging system, HPLC, MALDI TOF, Quadrupole ion trap mass spectrometry, Ion GeneStudio S5 next-generation sequencing (NGS) system, Beckman Coulter CytoFLEX, PacBio Onso platform, and Leica Live Cell Imaging system.
The department currently comprises seven research scientists with expertise across various domains and six scientific assistants. Fifteen PhD students are registered under the University of Madras and are actively pursuing their research within the department.
As the research arm of the Cancer Institute, we are committed to conducting high-quality, interdisciplinary research that contributes meaningfully to the fight against cancer.
Please feel welcome to contact our friendly reception staff with any general or medical enquiry call us.
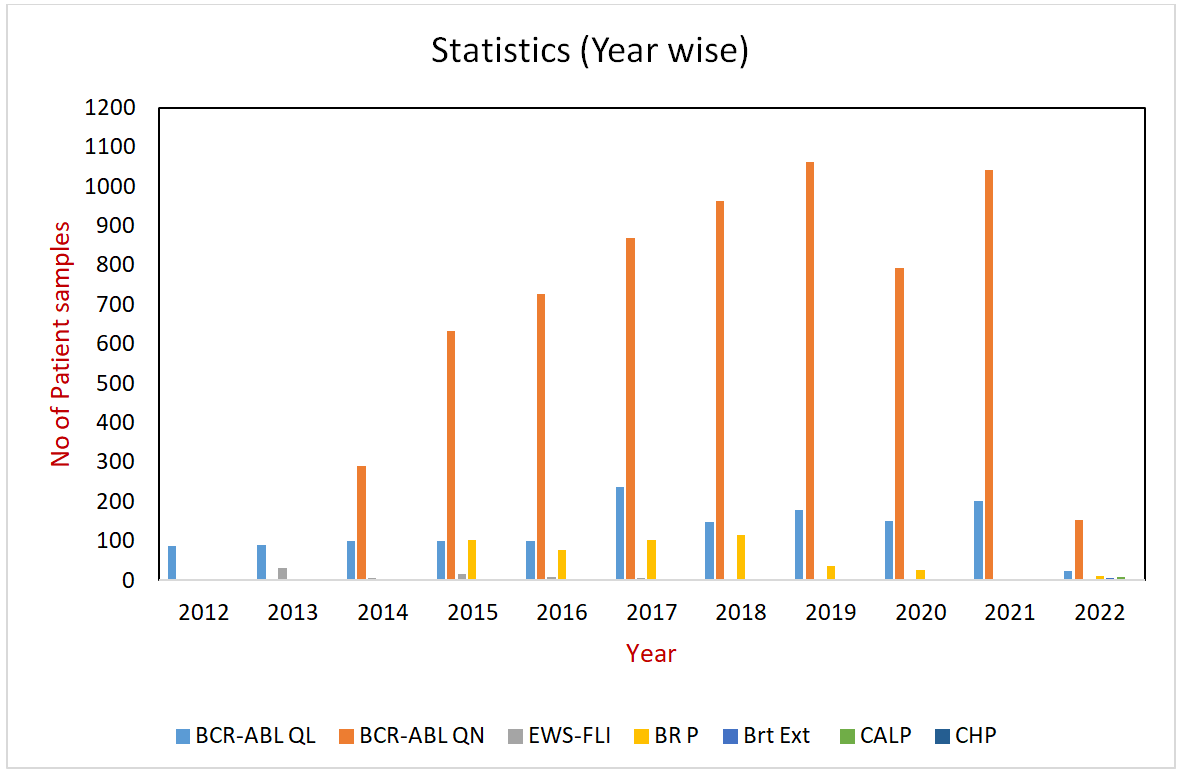
S.NO | TEST PARAMETERS | PLATFORM USED |
1 | BCR-ABL Qualitative Analysis | Real-Time PCR |
2 | BCR-ABL Quantitative Analysis | Real-Time PCR |
3 | EWS-FLI Testing | Reverse Transcriptase-PCR |
4 | BRCA1, BRCA2 & TP53 Gene Panel | Next Generation Sequencing |
5 | Breast Cancer Extended Gene Panel | Next Generation Sequencing |
6 | Cancer Hotspot Gene Panel | Next Generation Sequencing |
7 | Colon & Lung Cancer Gene Panel | Next Generation Sequencing |
8 | Lung RNA Fusion Gene Panel | Next Generation Sequencing |
| Mr. S. Jayavelu | MSc | Scientific Assistant |
| Mr. M. Vijayavel | Diploma, Animal Health Technology | Technologist – Animal House Facility |
| Ms. T. Sangeetha | MSc | Technician (Molecular Diagnostics) |
| Ms. S. Srividya Limkar | MSc | Technician (Molecular Diagnostics) |
| Mr. P. Pitchai Muthu | Diploma, Medical Laboratory | Lab Technician |
| Mr. M. Vijayavel | Diploma, Animal Health Technology | Technologist – Animal House Facility |
| Ms. S. Vaishnavi | MTech | Senior Research Fellow (Thesis Guide: Dr. T. Rajkumar) |
| Ms. R. Aadhithya | MTech | Junior Research Fellow (Thesis Guide: Dr. G. Gopal) |
| Ms. Keerthana Reddy | MSc | Junior Research Fellow (Thesis Guide: Dr. R. Balaji) |
| Ms. R. Nandhini | MBA | Social Investigator (Project PI: Dr. T. Rajkumar) |
2. Priya Ramanathan, Selvaluxmy Ganeshrajah, Rajalekshmi K.R, Shirley Sundar Singh, Thangarajan Rajkumar (2014). Development and clinical evaluation of dendritic cell vaccines for HPV related cervical cancer-a feasibility study. Asia Pacific J Cancer Prev. 15: 5909-5916.
3. Hascitha J, Priya R, Jayavelu S, Dhandapani H, Selvaluxmy G, Sunder Singh S, Rajkumar T. Analysis of Kynurenine/Tryptophan ratio and expression of IDO1 and 2 mRNA in tumour tissue of cervical cancer patients. Clin Biochem. 2016 Aug;49(12):919-24
4. Amutha Periyasamy, Gopal Gopisetty, Velusami Sridevi, Joyimallaya Subramanium Malliga, Thangarajan Rajkumar. Identification of candidate biomarker mass (m/z) ranges in Serous Ovarian Adenocarcinoma using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight mass spectrometry profiling. Biomarkers 2015 20(5):292-8.
5. Amutha Periyasamy and Thangarajan Rajkumar. Role of insulin-like growth factor, insulin-like growth factor receptors and insulin-like growth factor binding proteins in ovarian cancer. IJMPO, 2017; 38(2):198-206



Please feel free to contact our friendly staff with any medical enquiry.