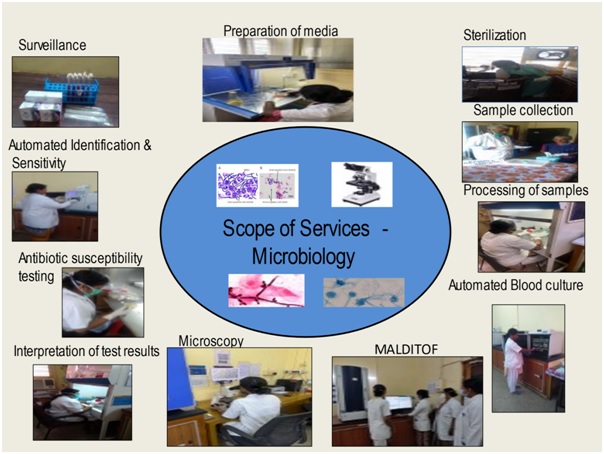
The microbiology department provides comprehensive bacteriological, fungal, mycobacterial culture and serological diagnostic service helping in treatment of patients with infection.
The microbiology department provides comprehensive bacteriological, fungal, mycobacterial culture and serological diagnostic service helping in treatment of patients with infection. A wide range of samples like blood, body fluids, urine, pus, respiratory secretions, pre op swabs, catheter tips, tissues, stool etc. are received for culture, around 40,000 clinical samples and around 12,000 surveillance samples per year.
The department has BacT alert automated systems – 120 cells for blood culture specific for adults & paediatric patients thereby helping reduce the turnaround time and results being available after a shortened incubation period of four hours instead of the conventional 24 hours, Mycobacterial culture done using BacT alert automated systems – 60 cells, Automated Vitek 2 Compact system used for identification and susceptibility testing of bacteria and fungus. Anaerobic, Fungal, and Mycobacterial culture of various clinical samples are routinely performed.
MALDI TOF is introduced for early identification of bacteria in blood cultures, urinary tract infections (UTIs), cerebrospinal fluids, respiratory tract infections, stool samples etc. The MALDI Biotyper System identifies microorganisms using MALDI-TOF (Matrix-Assisted Laser Desorption / Ionization Time of Flight) mass spectrometry to determine a unique proteomic Mass fingerprint of an organism.
The department has been helping in monitoring antibiotic resistance, preventing spread of infections, actively involved in surveillance of theatres, Robotic OT, ICU’s, BMT unit, various wards, CSSD, health care personnel and biomedical waste management for better infection control & conducts continued education for nursing and paramedical staff.
It is a major responsibility of the department, regular review of infections and antibiotic policy to limit the spread of infections & resistance is being done. Following the Institute policy of providing a safe hospital environment for our patients, we ensure adherence of strict infection control practice at all levels of the hospital. Relevant cultures are sent at the first suspicion of infection to identify focus and antibiotic initiation is done after sending cultures. Compliance with antibiotic policy is reviewed periodically & all efforts are made to reduce overall antibiotic use. As part of the NABH journey, we have initiated many quality control activities, all the NABH standards pertaining to the laboratory & Infection Control are being adhered at the department. Quality objective set to improve the Hand hygiene compliance from 70% to 80%. WHO – Infection prevention and control assessment tool & Hand hygiene self-assessment framework are being followed. Surveillance of CLABSI, VAP, SSI & CAUTI is done.
Please feel welcome to contact our friendly reception staff with any general or medical enquiry call us.
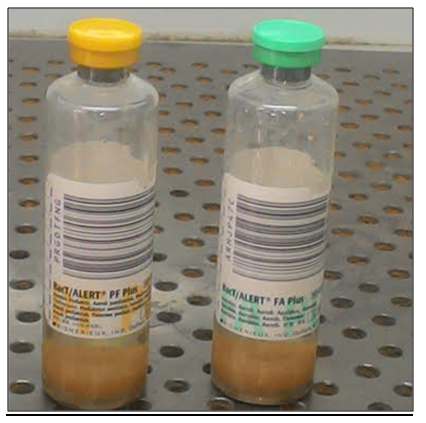
a qualitative Microbial detection system used for enhanced recovery and detection of aerobic and facultative microorganisms (Bacteria & fungi) from Blood.


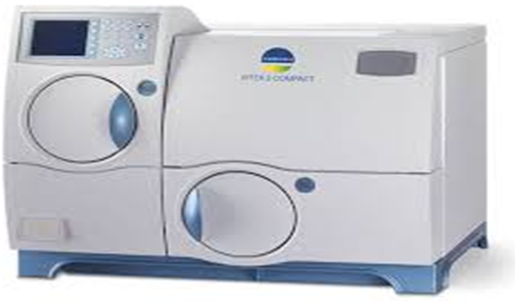

The VITEK 2 Compact system is dedicated to the identification of bacteria and yeasts and susceptibility testing of clinically significant bacteria.
The system includes the VITEK® 2 Compact instrument, a computer (workstation), and printer.
The software provided with the VITEK® 2 Compact system includes analysis and data-management programs. A bidirectional computer interface transfers results automatically to the user’s laboratory information system (LIS) and to various product and patient reports.
A Quality Control System is available to validate a VITEK® 2 Compact system test kit. An Advanced Expert System™ (Clinical Use) is available to provide online, systematic validation of results and interpretation of resistant phenotypes found during susceptibility testing.
Vitek 2C is automated bacterial and fungal identification and susceptibility system. This is an automated microbiology system utilizing growth-based technology, reducing hands on time for enhanced workflow & rapid reporting. [read more]
The reagent cards have 64 wells that contain an individual test substrate. Substrates measure various metabolic activities such as acidification, alkalization, enzyme hydrolysis, and growth in the presence of inhibitory substances.
An optically clear film present on both sides of the card allows appropriate level of oxygen transmission while maintaining a sealed vessel that prevents contact with the organism-substrate admixtures. Each card has a pre-inserted transfer tube used for inoculation. Cards have bar codes that contain information on product type, lot number, expiration date and a unique identifier that can be linked to the sample either before or after loading the card onto the system.
Inoculated cards are passed by a mechanism, which cuts off the transfer tube and seals the card prior to loading into the carousel incubator. The carousel incubator can accommodate up to 30 cards. All card types are incubated on-line at 35.5 + 1.0ºC. Each card is removed from the carousel incubator once every 15 minutes, transported to the optical system for reaction reading and then returned to the incubator until the next read time. Data are collected at 15-minute intervals during the entire incubation period. [/read]


Human coronaviruses can remain infectious on surfaces for up to 9 days. COVID-19 virus has been detected after up to 72 hours on plastic and steel, 24 hours on cardboard, 4 hours on copper therefore, cleaning the environment is paramount. Cleaning environmental surfaces with water and detergent and applying commonly used hospital disinfectants (such as sodium hypochlorite) is an effective and sufficient procedure. The following cleaning & disinfection protocol for control & prevention of spread of COVID 19 is strictly being followed in OPD’s, wards, high risk areas – ICU/OT, counter’s, other patient care areas & diagnostic laboratories at both campus.
Many disinfectants are active against enveloped viruses, such as the COVID-19 virus, including commonly used hospital disinfectants.[read more] Sodium hypochlorite & Lysol which is 50% cresol & 50% liquid soap is recommended for spraying. Floor Cleaning with 3 bucket mopping system is followed. High touch surface like doorknobs, door handles, handrails, patient chair rests & arms, lift doors & buttons, sink taps and other fixtures are cleaned by spraying 2.5% Lysol, 70% Isopropyl alcohol to be used to wipe down surfaces where the use of bleach/Lysol is not suitable. Disinfection to be done every 4 hours or more frequently. *Fogging is done using hydrogen peroxide+silver ion MIKROZID HP10 solution after every case & at the end of the list. Cleaning using automated wet mopping machine is done in weekends in above high-risk areas.
Detergent disinfectant B1, B5, B6 products & Harpic are used for toilets, removal of tough stains grease, ramp & corridor washing. Bleaching powder is sprinkled, & 1% hypochlorite is sprayed liberally for disinfection of General waste shed, Bio Medical waste shed & near all manhole, drain holes, drainpipes etc. The commonly used hospital disinfectants Diversy product benzalkonium chloride for floor mopping (Expert/ Swipol) are used first to remove the gross dust & dirt followed by 1% Sodium hypochlorite, 5% Lysol is sprayed in all areas. Pest control is done regularly in external/internal premises of the hospital. Guidelines for handling, treatment, and disposal of COVID-19 Bio Medical Waste are strictly followed.
Collecting & handling of laboratory specimens from patients with suspected COVID-19
All specimens should be regarded as potentially infectious & staff should adhere rigorously to the standard precaution measures and bio safety practices to minimize the possibility of exposure. Soiled linen not for reuse is placed in clearly labelled, leak-proof yellow bags. Decontamination of ambulance, routine cleaning of patient transport bus & staff transport bus regularly done. Disinfection of sump water with chlorination using bleaching powder was also done in regular intervals
Pharmacy: Frequent disinfection of the counter was performed. It was ensured that Attenders / patient wore mask at all times. Training: Training of doctors, nurses, and all Hospital Staff for adapting to current protocols was made mandatory & ongoing.
The department was part of organising Covid Vaccination Camp. [/read]

| 1 | Dr Packia Nancy | Assistant Professor & HOD | |
| 2 | Mrs. Varalakshmi | (M.Sc Medical Micro) | Head, Infection Control Officer, Associate Director(Admin) |
| 3 | Mrs. Harini | (M.Sc Medical Micro) | Scientific Assistant |
| 4 | Mrs. Saraswathy | (M.Sc Applied Micro) | Scientific Assistant |
| 5 | Ms. Samudhra | (MSc Medical Micro) | Junior Scientific Assistant |
| 6 | Mrs. Meena | (B.Sc MLT) | Technician |
| 7 | Mr. Vijayaragavan | (DMLT) | Technician |
| 8 | Ms. Monika | (DMLT) | Technician |
| 9 | Ms. Nandhini | (B.Sc, Applied Micro) | Trainee Technologist |
| 10 | Ms. Aarthishwari | (DMLT) | Trainee Technician |
| 11 | Ms Enisha | (B.Sc, Applied Micro) | Technologist |
Call: 044-22209150/22350131 Ext 105/152/164/169/211 between 7AM and 5PM
Please feel free to contact our friendly staff with any medical enquiry.